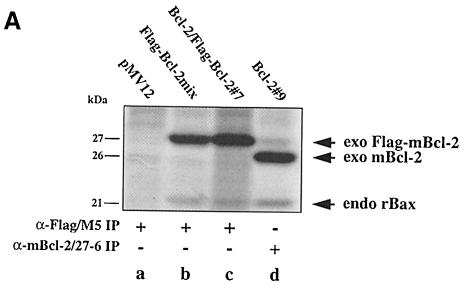
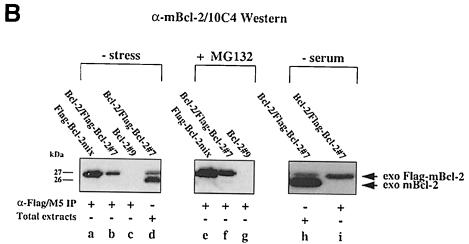
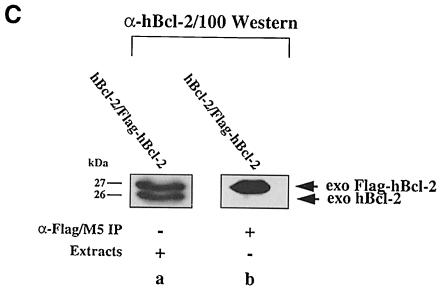
Fig. 3. No interaction between Bcl–2 and Flag-tagged Bcl–2 in R6 extracts. (A) α–Flag/M5 (lanes a–c) or α–mBcl–2/27–6 (lane d) immunoprecipitates from radiolabeled R6 cell extracts harboring either the transfer vector (pMV12) (lane a), Flag–mBcl–2 (R6-Flag–Bcl–2mix) (lane b), mBcl–2 (R6-Bcl–2#9) (lane d) or both (R6-Bcl–2/Flag–Bcl–2#7) (lane c). Note the absence of a band at 26 kDa in lane c, indicating that 26 kDa mBcl–2 does not co-precipitate with 27 kDa Flag–mBcl–2. (B) α–mBcl–2/10C4 Western blot of α–Flag/M5 immunoprecipitates from unlabeled extracts of R6 cells overexpressing Flag–mBcl–2 (R6-Flag–Bcl–2mix) (lanes a and e) or mBcl–2 (R6-Bcl–2#9) (lanes c and g) or both (R6-Bcl–2/Flag–Bcl–2#7) (lanes b, f and i) before (– stress) and after treatment with 1 μM MG132 (+ MG132) or depletion of serum for 48 h (– serum). Total extracts from R6-Bcl–2/Flag–Bcl–2#7 cells in the presence or absence of serum are shown in lanes d and h, respectively. Note that neither lane b nor lanes f and i contain co-precipitating 26 kDa exo mBcl–2 although it is clearly present in the total extracts (lanes d and h). (C) α–hBcl–2/100 Western blot of an extract (lane a) or an α–Flag/M5 immunoprecipitate of this extract (lane b) prepared from HEK cells co-transfected with Flag–hBcl–2 and hBcl–2. Note that the human forms of Bcl–2 also do not interact.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.