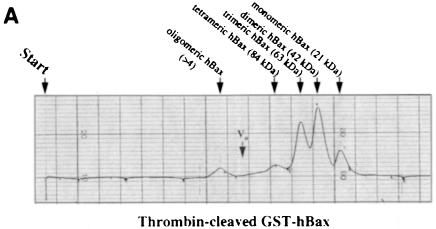
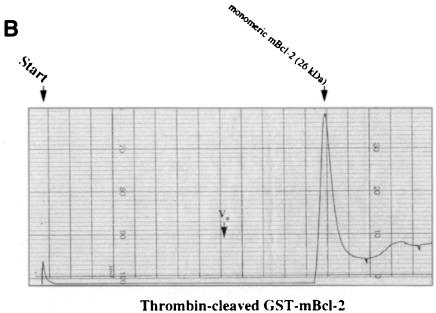
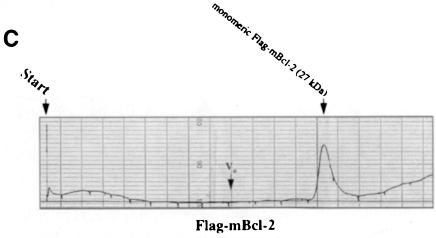
Fig. 4. Recombinant Bax, but not Bcl–2 or Flag–Bcl–2, forms multimers in vitro. On-line 280 nm measurements of glutathione affinity-purified, thrombin-cleaved GST–hBax (A), GST–mBcl–2 (B) or α–Flag/M2-purified Flag–mBcl–2 (C) eluting from an FPLC Superose 12 column. The time of sample application (Start) and the void volume of the column (Vo) are indicated. While hBax elutes in multiple peaks corresponding to the molecular masses of its multimers (21, 42, 63, 84 kDa and higher), mBcl–2 and Flag–mBcl–2 are recovered in one single peak each (monomeric 26 or 27 kDa forms, respectively). The molecular masses were calculated according to the elutions of the standard proteins carbonic anhydrase (29 kDa), ovalbumin (45 kDa), bovine serum albumin (66 kDa) and β–galactosidase (115 kDa).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.