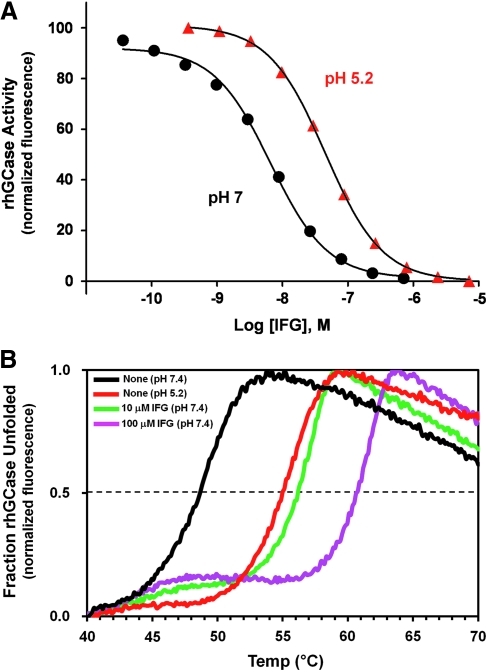
Fig. 3.
Enzyme inhibition and thermal stability assays can be used to characterize PCs for lysosomal enzymes. (A) Inhibition of recombinant human acid β-glucosidase (rhGCase) activity at pH 7 (endoplasmic reticulum pH) and pH 5.2 (lysosomal pH) as a function of IFG concentration. Inhibition of rhGCase activity by IFG was measured with the fluorogenic substrate 4-methylumbelliferyl-β-D-glucopyranoside (Sigma-Aldrich, St. Louis, MO). As seen in the enzyme inhibition curves, IFG binds with approximately sixfold higher affinity to rhGCase at neutral pH (circles) compared with acidic pH (triangles). (B) Thermal stability scans of rhGCase in the absence and presence of increasing concentrations of IFG. The unfolding of rhGCase was monitored using SYPRO Orange (Sigma-Aldrich). Binding of SYPRO Orange to exposed hydrophobic regions of a denatured protein results in increased fluorescence. rhGCase is physically more stable at acidic pH 5.2 (purple line) compared with neutral pH 7.4 (black line) as evident by a higher melting temperature at the lower pH. Likewise, as the concentration of IFG is increased at pH 7.4, rhGCase becomes more resistant to thermal denaturation (10 μM IFG, red line; 100 μM IFG, green line).