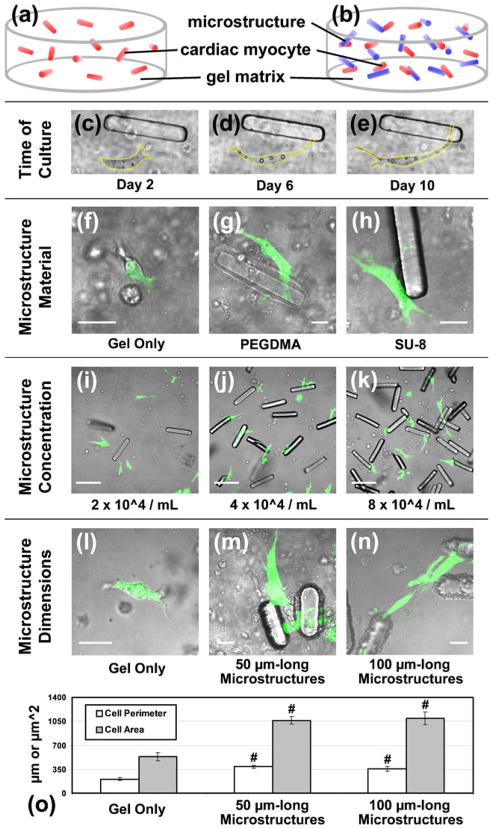
Fig. 1.
Microstructure optimization. Isolated neonatal rat ventricular myocytes were suspended with random orientation in a supporting 3D gel matrix (Matrigel) (a) without or (b) with engineered 100 μm-long microstructures (c–e) and cultured for 10 days while observed with phase microscopy (yellow line = cell boundary). (f) Live myocytes (green with calcein AM) in gel only or making contact with microstructures fabricated from either (g) poly (ethylene glycol) dimethacrylate (PEGDMA) or (h) SU-8 photo-resist (scale = 20 μm). (i–k) The concentration of microstructures in 3D gels was varied, with 4×104/mL yielding a single microstructure within approximately 100 μm of each cell (scale = 100 μm). Myocyte morphology in (l) gel alone or with (m) 50× 15×15 or (n) 100×15×15 μm3 microstructures in 3D (scale = 20 μm). (o) The average perimeter and area of live myocytes is significantly lower in control gels than with 50 and 100 μm-long microstructures, but cell morphology across the two microstructure groups is similar (n≥8). Mean ± SEM. #p<0.01