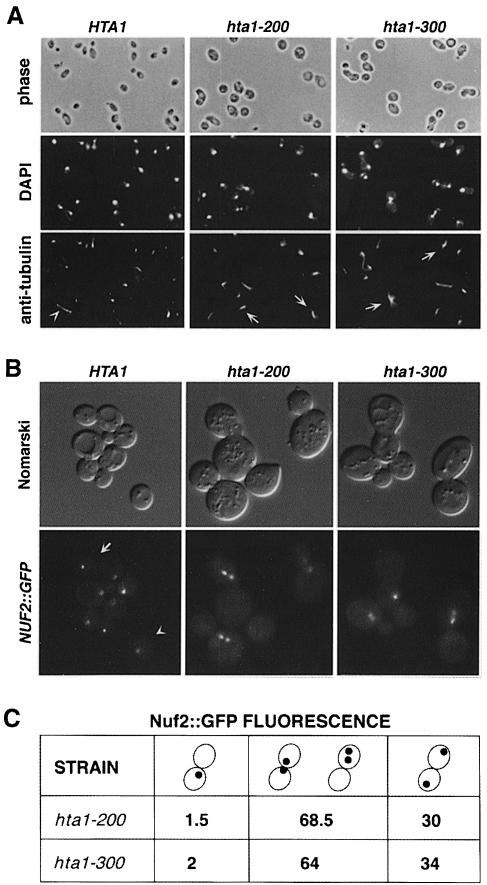
Fig. 5. Microtubule and spindle pole body analysis in synchronized cells shifted to 13°C. (A) Microtubule staining by indirect immuno- fluorescence with anti-tubulin antibodies of cells taken 24 h after the shift to 13°C. Nuclear DNA was stained with DAPI. The hta1 mutant shows large-budded cells with an undivided nucleus and a short spindle. The arrowhead indicates a wild-type cell in anaphase with an elongated spindle. Arrows indicate hta1 large-budded cells with a short spindle. Strains used were: HTA1 (FY605), hta1–200 (FY1817) and hta1–300 (FY1818). (B) Spindle pole body staining by green fluorescence on live cells marked with NUF2::GFP. Cells were synchronized with α–factor and released at 13°C; a sample was taken 24 h after the shift to 13°C and analyzed by fluorescence microscopy. In the wild-type cells a single dot corresponding to an undivided spindle pole body is present in an unbudded cell (arrowhead), or two dots at the opposite poles of a large-budded cell (arrow) are present in a cell at anaphase. The two dots shown in mutants correspond to the spindle pole body that has duplicated and separated a short distance within a large-budded cell, consistent with a large-budded cell with an undivided nucleus. In some cases only one dot appears in the focal plane of the picture. (C) Quantitation of the GFP signal in the hta1–200 and hta1–300 strains. Numbers represent percentage values. Strains used were: HTA1 (FY1825), hta1–200 (FY1826) and hta1–300 (FY1827).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.