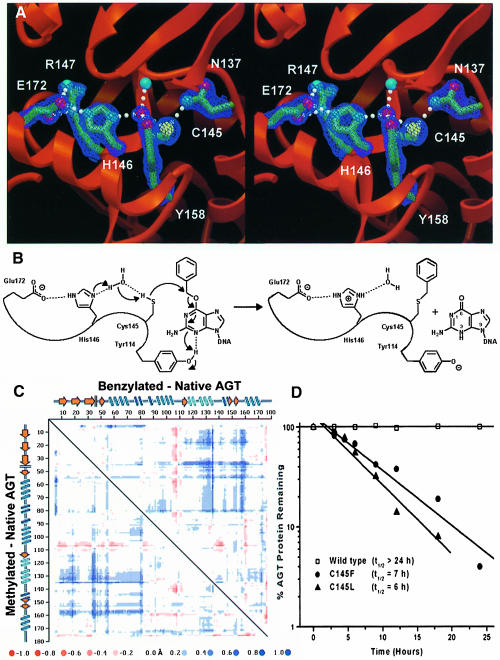
Fig. 5. AGT active site structure, dealkylation mechanism and stability of mutants mimicking alkylated AGT. (A) Active site hydrogen bond network, required for both stability and activity. Electron density from a σA-weighted 2Fo – Fc map contoured at 2σ is shown. (B) A proposed reaction mechanism for AGT. His146, acting as a water-mediated general base, deprotonates Cys145 to facilitate attack at the O6–alkyl carbon, with concomitant protonation of N3 by Tyr114. (C) Distance difference matrix plot of benzylated (upper right) and methylated (lower left) versus native AGT showing 0.5–1.5 Å shifts of helix H6 (residues 125–136) and the guanine-binding loop (residues 153–160) away from the N-terminal domain. This opening of the tertiary structure, accommodating the alkyl adducts, distorts the DNA-binding surface. (D) Instability of Cys145 mutants. Wild-type and C145F and C145L mutants were expressed in E.coli lacking endogenous AGT. Following arrest of protein synthesis, the presence of AGT was measured as a function of time by immunoblotting of cell lysates with anti-AGT antibodies. C145F and C145L mutants, which mimic alkylated C145, demonstrate in vivo instability.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.