Abstract
BACKGROUND:
Little is known about the nature and the course of IgA nephropathy (IgAN) in Arab countries. The aim of this work was to study the spectrum of clinical presentation and histopathological findings at our institution.
DESIGN AND SETTING:
Retrospective review, all renal biopsies at the Mubarak Al Kabeer Hospital between January 2000 and December 2004.
METHODS:
Cases of IgA nephropathy were selected, and their medical records and biopsy findings were reviewed.
RESULTS:
Eighty patients (9.2% of all native kidney biopsies) were diagnosed to have IgAN nephropathy. Sixty-nine biopsies were included in the study;11 were excluded. Forty-three (62.3%) patients were male and 26 (37.7) patients were female. Fifty (72.5%) patients were below the age of 40 years. Mean (SD) duration of follow-up was 3.6 (1.3) years. The first presentation included nephritic-range proteinuria (49.3%) and renal impairment (50.7%). During the follow-up period, 56 (81.2%) patients were stable or improved. Hass classification of biopsies showed 36.2% had class I, 27.5% had class II, 13.0% had class III, 5.8% had class IV, and 17.4% had class V IgAN. Females had milder forms of the disease than males. Macroscopic hematuria and renal impairment at presentation were seen more in patients with class IV and V IgAN. The presenting serum creatinine and uric acid values were higher in those with Hass classes III to V. Deterioration of renal function during the follow-up period was more significant in the presence of hypertension, renal impairment, or macroscopic hematuria at the time of biopsy .
CONCLUSION:
The prevalence of IgAN in Kuwait is about 9.2%. Renal impairment or macroscopic hematuria at presentation was seen in patients with more aggressive renal lesions and contributed to poor outcome.
IgA nephropathy (IgAN) was first described in 1968 by Berger and Hinglais.1 It is now recognized as the most common primary glomerulonephritis worldwide.2 It presents with hematuria and often proteinuria. Although a moderate degree of proteinuria is common in patients with IgAN, nephrotic syndrome is considered uncommon in these patients.3 The course of IgAN is variable, and 15% to 40% of patients progress to end-stage renal disease over a period of 10 to 20 years.4 The pathogenesis of IgAN is complex and not completely understood. Both environmental and genetic factors have been found to be involved in the disease onset and progression.4,5 Humoral immunity is believed to play an important role, characterized by the predominant mesangial IgA1 deposition and associated secondary inflammatory response.5 Therapeutic efforts have been directed at either reducing or preventing antigen entry, as well as altering the abnormal immune response and its consequences. However, the appropriate therapy for IgAN remains uncertain, and curative therapy is still not available.6,7 The aim of this study was to review cases of IgAN treated at our institution over a 5-year period and to study the spectrum of clinical presentation and histopathological findings.
METHODS
All renal biopsies performed at the Mubarak Al Kabeer Hospital from January 2000 to December 2004 were retrospectively reviewed. Biopsies performed on adult patients with IgAN were selected and reviewed. Patients were excluded from the study if clinical or serologic evidence of Henoch Schonelin purpura, collagen vascular diseases, liver cirrhosis, diabetes mellitus, or other kidney diseases was present. Kidney transplant cases were also excluded from the study. Clinical and laboratory data at presentation and during the follow-up period and the details of treatment were obtained by careful retrospective study of the hospital records of each patient.
The histopathology glass slides were reviewed, and the pathology reports were retrieved from the Department of Pathology computerized filing system. Each kidney biopsy was prepared by cutting paraffin blocks into 3-μm sections and staining 2 slides with periodic acid Schiff, 2 slides for hematoxylin and eosin, 1 slide for Jones methenamine silver and 1 slide for trichrome. Immunoperoxidase staining was also performed routinely on all slides for IgG, IgA, IgM and C3. Antibodies were from Dako, and titration was performed according to the leaflets with the antibody vials. Electron microscopy (EM) was not routinely done in all cases in the institution; however, in selected cases, EM was performed and the films were retrieved and reviewed along with the EM report.
Numerical variables are expressed as mean (standard deviation). The relationships within and between the clinical and the histopathological variables were obtained using the chi-square test or Fisher exact probability test for categorical variables and nonparametric Mann-Whitney U test and Kruskal-Wallis test for continuous variables. P less than .05 was considered to be statistically significant. Statistical analysis was performed using SPSS for Windows version 16 (SPSS, Inc., Chicago, IL).
RESULTS
During the 5-year period, 1575 renal biopsies were performed with 871 biopsies on native kidneys and 704 on transplanted kidneys. Eighty patients (representing 9.2% of the native kidney biopsies, 5.1% of the total biopsies) were found to have IgA nephropathy according to the biopsy results. Eleven patients were excluded from the study because of missing data or the presence of any of the exclusion criteria. Sixty-nine patients were enrolled in the study (Table 1). Serum IgA, C3, and C4 levels were all within the normal reference range. Serum antinuclear, anti-DNA double-stranded, anti-glomerular basement membrane and anti-neutrophil cytoplasmic antibodies were all negative.
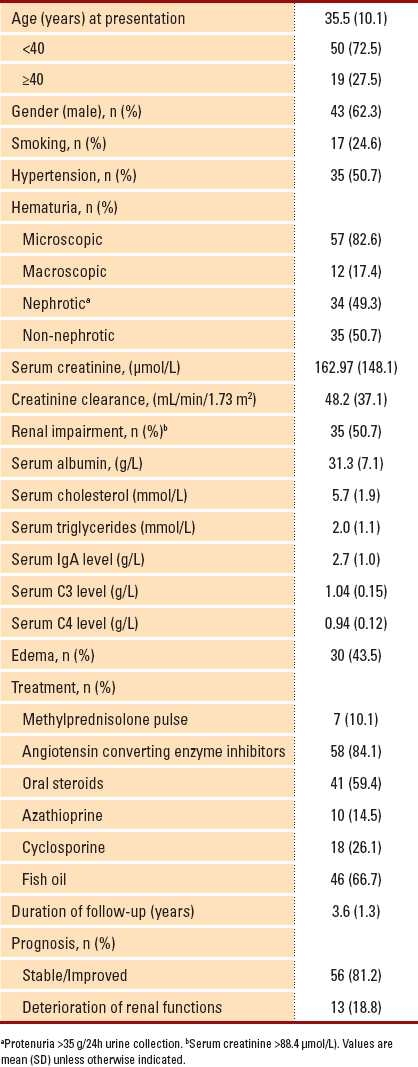
Table 1.
Clinical and laboratory data of patients having IgA nephropathy (n= 69).
Evaluation of renal biopsy slides was performed according to the Hass classification of IgA nephropathy (Table 2).8 All tissues were found to stain negative for IgG, IgM and C3 (Figures 1, 2). Seven (10.4%) patients were treated with pulse methylprednisolone for crescentic lesions that presented as acute renal failure with no evidence of pulmonary hemorrhage or systemic vasculitis; 41 (59.4%) patients were treated with oral corticosteroids; 10 (14.5%) received mycophenolate mofetil or azathioprine; 18 (26.1%) received cyclosporine; and 58 (84.1%) patients were treated with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers. Fish oil was given as an adjuvant therapy in 46 (66.7%) cases. Females had a milder histological form of the disease (class I), whereas males tended to have more aggressive forms (classes IV and V) (P<.05) (Table 3). No relationship was found between the Hass classification and age at presentation, hypertension, presence of edema or the level of proteinuria (P>.05). Macroscopic hematuria was seen more in patients with class IV (75%) and class V (25%) IgAN than in patients with class I (8%) IgAN (P<.05). Renal impairment at presentation was seen more in patients with class IV (75%) and class V (91%) IgAN than in those with class I (28%) IgAN (P<.001). The levels of presenting serum creatinine and uric acid were higher in those with Hass classes III to V than in those with classes I and II (P<.001 and <.05, respectively).
Table 2.
Histopathological spectrum of renal biopsy results according to Hass classification among IgAN patients (n=69).
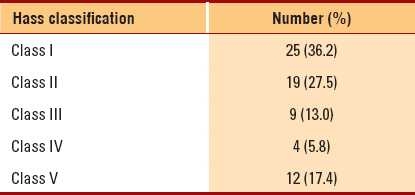
Figure 1.
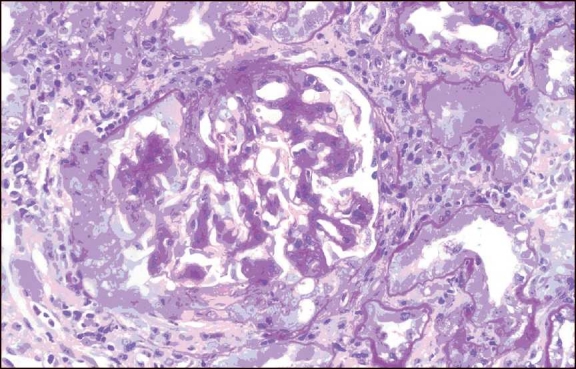
A case of crescentic IgA nephropathy. Mesangial expansion with a cellular crescent (PAS, ×400)
Figure 2.
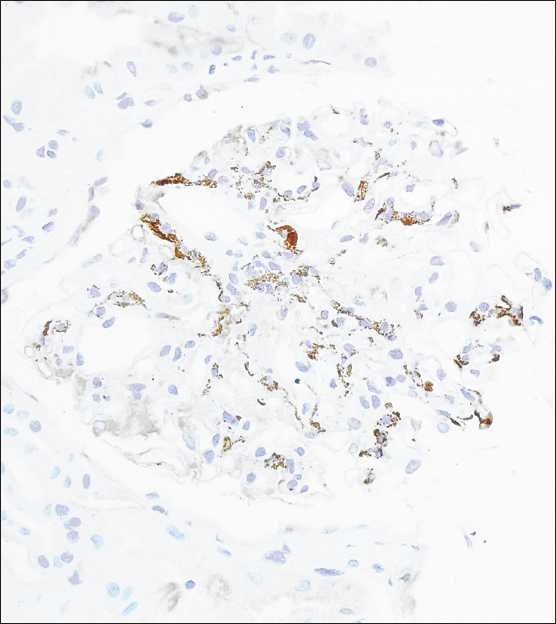
Immunoperoxidase staining shows a prominent mesangial pattern (IgA immunoperoxidase, ×400)
Table 3.
Relationship between clinical presentation and Hass classification (n=69).

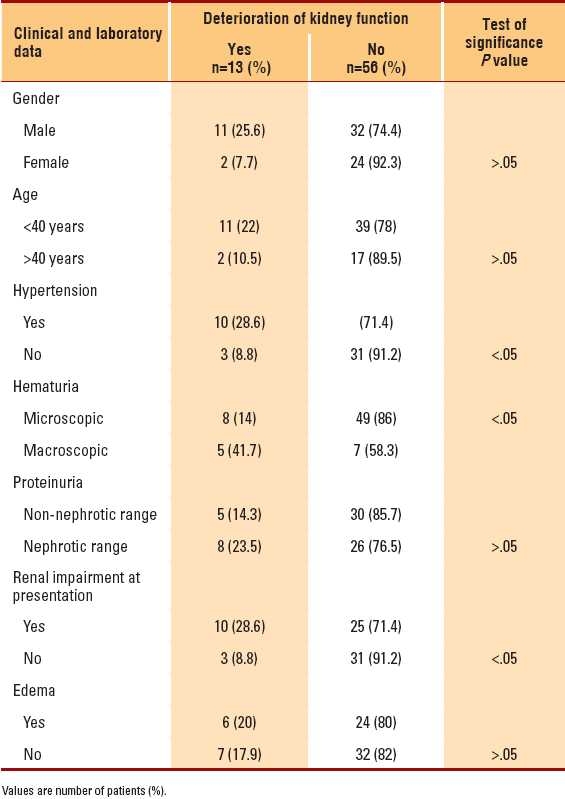
Deterioration of renal function during the follow-up period was more significant in the presence of hypertension, renal impairment at time of biopsy, and macroscopic hematuria (P<.05) (Table 4), whereas the presenting levels of proteinuria, age, gender and Hass classification had nonsignificant effect on the deterioration of kidney functions (P>.05). The higher the level of presenting serum creatinine, the more the deterioration of renal function during the follow-up period (P< .05).
Table 4.
Factors determining worsening of the kidney function during the follow-up period (n=69).
DISCUSSION
Many reports of glomerulonephritis associated with mesangial IgA deposits have been published since the original report on IgAN by Berger and Hinglais.1 The apparent incidence of this disorder has varied in studies from different countries. In France,9 Spain,10 Japan11 and Italy,12 the incidence has ranged from 11.7% to 43.3% of the total number of renal biopsies. Much lower incidences have been reported in the Unites States,13 England14 and Canada,15 with the incidence ranging from 2.0% to 8.5% in these countries. Berger16 suggested that the higher reported incidence of this disease in certain countries compared to others may reflect the practice of routine annual urinalysis in the countries with high incidence rates. To the best of our knowledge, this is the first study in an Arab country demonstrating the incidence of IgAN. We reported the incidence to be 9.2% of native kidney biopsies in Kuwait. Since the original description of IgAN, a number of studies have attempted to correlate initial clinical and pathological findings with the subsequent course of the disease. The results of the present study are in accordance with those of the previous studies in showing that females had milder pathologic changes whereas males were shown to have more aggressive forms.17 A difference in the incidence of macroscopic hematuria in adult patients was found when compared between various geographical regions.18 In European countries, the reported incidence exceeded 50%;19,20 whereas in Japan, the incidence ranged from 15% to 31%.21,22 This difference in distribution can be attributed to the difference in the nature of the disease, which could be linked to genetic factors.19 The prognostic significance of macroscopic hematuria was found to be controversial. In the present study, macroscopic hematuria was detected in 17.2% of cases. It was associated with aggressive histologic findings and correlated with a poor prognosis. This confirmed the results arrived at by the Southwest Pediatric Nephrology Study Group.17 Moreover, Bennet and Kinciad-Smith23 reported that renal function became significantly worse in those with macroscopic hematuria, and emphasized the high incidence of crescent formation in these cases. However, Clarkson et al24 demonstrated that renal function and lesions were significantly better in patients with macroscopic hematuria than in those without it. In our study, renal impairment at presentation was seen more in patients with class IV and class V IgAN than in those with class I IgAN. Correlations between more extensive pathologic features and severe clinical manifestation were also documented by Hass et al.25
The presenting serum uric acid levels correlated with the pathologic findings of higher levels in those with Hass classes III to V IgAN than in those with class I and II IgAN. This confirmed the results of the study by Myllimaki et al,26 who proved a strong correlation between serum uric acid level and severity of renal damage on biopsy.
The appropriate treatment for IgA nephropathy remains uncertain. No curative treatment is available.6,7 In the present study, pulse methylprednisolone therapy followed by oral corticosteroids plus azathioprine or cyclosporine was used in cases of crescentic glomerulonephritis with acute renal failure. On the other hand, oral immunosuppressive treatment was used in cases of mild-to-moderate mesangial proliferation and was withheld if there was any evidence of chronic changes (glomerulosclerosis or tubulointerstitial atrophy) in the biopsy specimen. Angiotensin-converting enzyme inhibitor and/or angiotensin-receptor blockers was used as an adjuvant nonspecific antiproteinuric measure in 84.1% of cases.
The overall prognosis of IgAN remains to be confirmed. In adult studies, the incidence of renal insufficiency is found to vary from less than 10% to 48% in patients followed for more than 1 year,27 which is in agreement with the result of the present study, in which renal insufficiency was seen in 18.8% of the cases. Bartosik et al28 proved that the clinical parameters, such as hypertension and severity of proteinuria, appear to be stronger prognostic indicators than histological findings. Moreover, Van Der Peer et al29 found that those with higher levels of hypertension and proteinuria and more marked histologic findings had greater deterioration of their renal functions during follow–up when compared with those with lower levels and less marked findings. Another study showed that females and younger patients were found to have a better prognosis.30 In the present work, deterioration of renal function during the follow-up period was more significant in the presence of hypertension, renal impairment at the time of biopsy, and macroscopic hematuria, whereas the presenting levels of proteinuria, age, gender and Hass classification had a nonsignificant effect on the deterioration of kidney function.
In conclusion, the prevalence of IgAN in Kuwait is 9.2%. A multicenter study should be conducted to detect the exact incidence. About 18.8% of patients had deterioration of their renal function during the study period, but a longer follow-up is needed.
REFERENCES
- 1.Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris) 1968;74:694–5. [PubMed] [Google Scholar]
- 2.Donadio J, Grande J. IgA Nephropathy. N Engl J Med. 2002;347:738–48. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 3.Eitner F, Ackermann D, Hilgers RD, Floege J. Supportive versus immunosuppressive therapy of progressive IgA nephropathy (STOP) IgAN trial: Rationale and study protocol. J Nephrol. 2008;21:284–9. [PubMed] [Google Scholar]
- 4.Bruce AJ, Jan N. IgA nephropathy: An update. Curr Opin Nephrol Hypertens. 2004;13:171–9. doi: 10.1097/00041552-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Baek JE, Chang JW, Min WK, Cho YM, Park JS, Kim SB. Serum high sensitivity C-reactive protein is not increased in patients with IgA nephropathy. Nephron Clin Pract. 2008;108:C35–40. doi: 10.1159/000112527. [DOI] [PubMed] [Google Scholar]
- 6.Tang S, Leung JC, Chan LY, Lui YH, Tang CS, Kan CH, et al. Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int. 2005;68:802–12. doi: 10.1111/j.1523-1755.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- 7.Frisch G, Lin J, Rosenstock J, Markowitz G, D’Agati V, Radhakrishnan J, et al. Mycophenolate mofetil (MMF) vs Placebo in patients with moderately advanced IgA nephropathy: A double-blind randomized controlled trial. Nephrol Dial Transplant. 2005;20:2139–45. doi: 10.1093/ndt/gfh974. [DOI] [PubMed] [Google Scholar]
- 8.Hass M. Histologic subclassification of IgA nephropathy: A clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–42. doi: 10.1016/s0272-6386(97)90456-x. [DOI] [PubMed] [Google Scholar]
- 9.Navas-Palacios JJ, Gutierrez-Millet V, Usera-Sárrage G, Garzón-Martin A. IgA nephropathy: An ultrastructural study. Ultrastruct Path. 1981;2:151–61. doi: 10.3109/01913128109064244. [DOI] [PubMed] [Google Scholar]
- 10.Seeman T, Pohl M, John U, Dusek J, Vondrák K, Janda J, et al. Ambulatory blood pressure, proteinuria and uric acid in children with IgA nephropathy and their correlation with histopathological findings. Kidney Blood Press Res. 2008;31:337–42. doi: 10.1159/000164800. [DOI] [PubMed] [Google Scholar]
- 11.Cook HT. Pathological predictors of prognosis in immunoglobulin A nephropathy: A review. Curr Opin Nephrol Hypertens. 2009;18:212–9. doi: 10.1097/MNH.0b013e328329605c. [DOI] [PubMed] [Google Scholar]
- 12.Katafuchi R, Ninomiya T, Mizumasa T, Ikeda K, Kumagai H, Nagata M, et al. The improvement of renal survival with steroid pulse therapy in IgA nephropathy. Nephrol Dial Transplant. 2008;23:3915–20. doi: 10.1093/ndt/gfn394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SM, Moon KC, Oh KH, Joo KW, Kim YS, Ahn C, et al. Clinicopathologic characteristics of IgA nephropathy with steroid-responsive nephrotic syndrome. J Korean Med Sci. 2009;24:S44–9. doi: 10.3346/jkms.2009.24.S1.S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakayama K, Ohsawa I, Maeda-Ohtani A, Murakoshi M, Horikoshi S, Tomino Y. Predictors of diagnosis of immunoglobulin A nephropathy prior to renal biopsy and correlation with urinary sediment findings and prognostic grading. J Clin Lab Anal. 2008;22:114–8. doi: 10.1002/jcla.20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballardie FW, Cowley RD. Prognostic indices and therapy in IgA nephropathy: Toward a solution. Kidney Int. 2008;73:249–51. doi: 10.1038/sj.ki.5002660. [DOI] [PubMed] [Google Scholar]
- 16.Berger J. Idiopathic mesangial deposition of IgA, in Nephropathy. In: Hamburger J, Crosnier J, Grunfeld JP, editors. New York: John Wiley and Sons; 1979. pp. 535–41. [Google Scholar]
- 17.A multicenter study of IgA nephropathy in children. A report of the South West Pediatric Nephrology Study Group. Kidney Int. 1982;22:643–52. [PubMed] [Google Scholar]
- 18.Shimaada H, Imai N, Miyakawa Y, et al. [AUTHOR: Please provide remaining author names] Clinical spectrum of IgA nephropathy.A clinicopathological study of adult Japanese IgA nephropathy patients: Early stage cases and cases with exacerbations. Nephrology. 1997;3:S701–7. [Google Scholar]
- 19.D’Amico G, Imbasciati E, Barbiano Di Belgioioso G, Bertoli S, Fogazzi G, Ferrario F, et al. IgA mesangial nephropathy: Clinical and histological study of 374 patients. Medicine. 1985;64:49–60. [PubMed] [Google Scholar]
- 20.Reich HN, Troyanov S, Scholey JW, Cattran DC. Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–83. doi: 10.1681/ASN.2007050526. [DOI] [PubMed] [Google Scholar]
- 21.Rositto AE, Cobenas C, Drut R. Linear IgA disease of childhood developing IgA nephropathy. Pediatr Dermatol. 2008;25:339–40. doi: 10.1111/j.1525-1470.2008.00677.x. [DOI] [PubMed] [Google Scholar]
- 22.Mitsuiki K, Harada A, Okura T, Higaki J. Histopathologically advanced IgA nephropathy treated successfully with prednisolone and cyclophosphamide. Clin Exp Nephrol. 2007;11:297–303. doi: 10.1007/s10157-007-0497-0. [DOI] [PubMed] [Google Scholar]
- 23.Bennett WM, Kincaid-Smith P. Macroscopic hematuria in mesangial IgA nephropathy: Correlation with glomerular crescents and renal dysfunction. Kidney Int. 1983;23:393–400. doi: 10.1038/ki.1983.32. [DOI] [PubMed] [Google Scholar]
- 24.Clarkson AR, Seymour AE, Thompson AJ, Haynes WD, Chan YL, Jackson B, et al. IgA nephropathy; a syndrome of uniform morphology, diverse clinical features and uncertain prognosis. Clin Nephrol. 1977;8:459–71. [PubMed] [Google Scholar]
- 25.Haas M, Rahman MH, Cohn RA, Fathallah-Shaykh S, Ansari A, Bartosh SM. IgA nephropathy in children and adults.Comparison of histopathologic features and clinical outcomes. Nephrol Dail Transplant. 2008;23:2537–45. doi: 10.1093/ndt/gfn014. [DOI] [PubMed] [Google Scholar]
- 26.Myllymäki J, Honkanen T, Syrjänen J, Helin H, Rantala I, Pasternack A, et al. Uric acid correlates with severity of histopathological parameters in IgA nephropathy. Nephrol Dial Transplant. 2005;20:89–95. doi: 10.1093/ndt/gfh584. [DOI] [PubMed] [Google Scholar]
- 27.Moura IC, Benhamou M, Launay P, Vrtovsnik F, Blank U, Monteiro RC. The glomerular response to IgA deposition in IgA nephropathy. Semin Nephrol. 2008;28:88–95. doi: 10.1016/j.semnephrol.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Bartosik LP, Lajoie G, Sugar L, Cattran DC. Predicting progression in IgA nephropathy. Am J kidney Dis. 2001;38:728–35. doi: 10.1053/ajkd.2001.27689. [DOI] [PubMed] [Google Scholar]
- 29.Van der Peet J, Arisz L, Brentjens JR, Marrink J, Hoedemaeker PJ. The clinical course of IgA nephropathy in adults. Clin Nephrol. 1977;8:335–40. [PubMed] [Google Scholar]
- 30.Roufosse CA, Cook HT. Pathological predictors of prognosis in immunoglobulin A nephropathy: A review. Curr Opin Nephrol Hypertens. 2009;18:212–9. doi: 10.1097/MNH.0b013e328329605c. [DOI] [PubMed] [Google Scholar]


