Abstract
A smooth and efficient oxidation of isonitriles to isocyanates by sulfoxides is catalyzed by trifluoroacetic anhydride. Using DMSO as the oxidant and 5 mol-% TFAA (dichloromethane, −60 °C to 0 °C), the process is complete in a few minutes, forming dimethylsulfide as the only byproduct. The newly-formed isocyanates may be used directly or isolated in high purity by solvent evaporation.
As part of a program to redirect intermediates in the Pummerer reaction of sulfoxides into useful new multicomponent processes, we serendipitously discovered a fast and gentle catalytic oxidation of isonitriles to isocyanates that promises to be of utility to synthetic chemists. Besides using inexpensive, readily available reagents, the method generates volatile and innocuous byproducts often making possible direct isolation of the desired isocyanate without extractive workup.
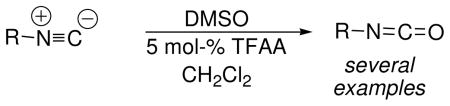
Given the broad synthetic utility of isocyanates,1 dozens of procedures have been described for preparing them, including several routes from isonitriles. Isonitrile-to-isocyanate oxidations have been reported using mercuric oxide,2 lead tetraacetate,3 and ozone,4 as well as halogen- or acid-catalyzed oxidations by dimethylsulfoxide (DMSO)5 and pyridine-N-oxide.6 However, recent interest in highly functionalized isocyanates drives the continuing demand for new synthetic methodology.7 Here we report that isonitriles are rapidly oxidized to isocyanates using DMSO in the presence of catalytic quantities of trifluoroacetic anhydride (TFAA, eq 1). Unlike the halogen-catalyzed DMSO oxidations reported earlier, which require prolonged heating at reflux and have been proposed to involve isonitrile-halogen adducts, the TFAA-catalyzed oxidations occur rapidly at low temperature by a different mechanism.
 |
(1) |
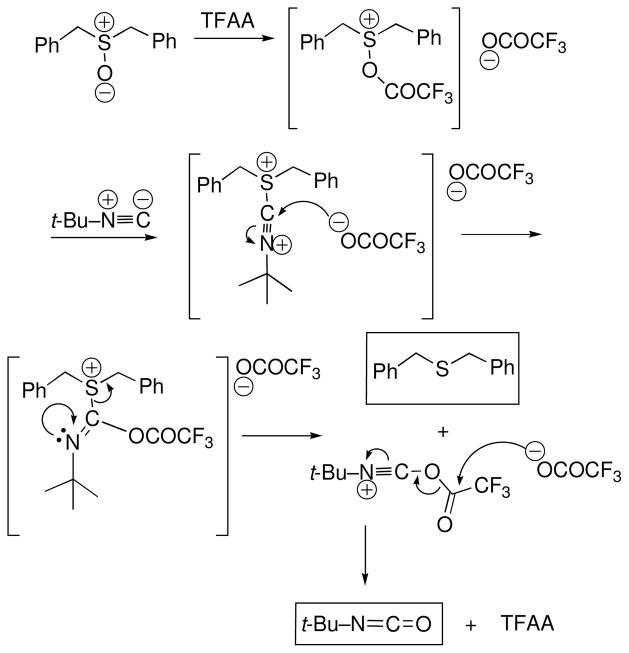
Initial attempts to generate and trap the putative sulfonium intermediates in Pummerer reactions led us to investigate the reaction of TFAA with dibenzyl sulfoxide in the presence of nucleophilic isonitriles such as t-BuNC (1:1:1 mixtures in CH2Cl2, 0 °C to rt, 10 min). In each case, the only isolable product obtained was dibenzylsulfide in near-quantitative yield.
Mechanistic considerations suggested that sulfoxide reduction might be accompanied by oxidation of the isonitrile either to the corresponding isocyanate R–N=C=O or its hexafluoroacylal R–N=C(OCOCF3)2. Real-time monitoring of the reaction mixture by IR unambiguously established the formation of isocyanate (2257 cm−1). A plausible mechanism for the overall redox process is shown in Scheme 1.
Scheme 1.
Proposed mechanism for the oxidation of isonitriles by sulfoxides
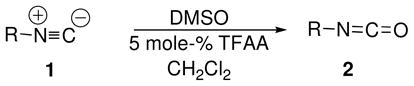
The proposed mechanism predicts that isocyanates should form using DMSO as oxidant and catalytic quantities of TFAA, although unlike the halogen-catalyzed DMSO oxidations of isonitriles reported earlier,5,6 the mechanism does not involve an isonitrile adduct with the catalyst. In accordance with Scheme 1, a sample of t-butylisonitrile was treated with DMSO (1.1 equiv) and TFAA (0.05 equiv) at low temperature (CH2Cl2, −60 °C to rt, 10 min), whereupon a strong isocyanate band was observed in the IR and trapping with t-butylamine afforded di-t-butylurea in 96% yield. The scope and generality of the method are indicated by results summarized in Table 1.
Table 1.
Oxidation of Isonitriles 1 to Isocyanates 2.
| isonitrile | isocyanate (% yield) | derivativea (% yield) |
|---|---|---|
| 1a R = t-Bu | 2a | (t-BuNH)2CO (96) |
| 1b R = n-Bu | 2b | n-BuNHCONH-t-Bu (95) |
| 1c R = p-CH3OC6H4 | 2c | p-CH3OC6H4NHCONH–t-Bu (61) |
| 1d R = β-morpholinoethyl | 2d | β-morpholinoethylNHCONH–t-Bu (67)b |
| 1e R = cyclohexyl | 2e (94) | — |
| 1f R = CH2CO2Et | 2f (95) | — |
Spectroscopic data for all derivatives matched literature values.
1.1 equiv TFAA was used in this experiment.
Since the only byproducts accompanying isocyanate formation, dimethylsulfide and residual TFAA, are volatile, it was of interest to determine whether some isocyanates might be isolated directly in nearly pure form by careful evaporative workup. Entries 5 and 6 in Table 1 show that cyclohexyl isocyanate 2e and ethyl isocyanatoacetate 2f can be isolated in excellent yields by rotary evaporation of solvent. In each case, the only byproduct detectable by NMR is a few percent of residual DMSO.8
The examples in Table 1 indicate that alkyl, cycloalkyl and aryl isonitriles were smoothly transformed into isocyanates using catalytic amounts of TFAA, with one exception. Oxidations of morpholinoethylisonitrile 1d using 5–20% TFAA returned substantial amounts of starting isonitrile.
To test whether amine/anhydride interactions interfered with catalysis, the oxidation was conducted using 1.1 equiv of TFAA. While the isonitrile was completely consumed, no characteristic stretching frequency for the isocyanate group in 2d was detected. Neverthteless, addition of t-butylamine to the crude product afforded the expected urea.9
In conclusion, the method reported here is simple and easy to use, and represents a very mild, rapid and environmentally acceptable procedure for preparing isocyanates from isonitriles. Not to be overlooked is the concomitant and equally useful synthetic conversion of sulfoxides to sulfides.
Supplementary Material
Acknowledgments
Funding was provided by the NIH (GM 008500 Training Grant Support for HVL). Support of the Cornell NMR Facility has been provided by NSF (CHE 7904825; PGM 8018643) and NIH (RR02002).
Footnotes
Supporting Information Available Representative experimental procedures as well as supporting spectroscopic data for the compounds described in Table 1. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Ozaki S. Chem Rev. 1972;72:457. [Google Scholar]
- 2.Gautier A. Ann Chim (Paris) 1869;17:229. [Google Scholar]
- 3.Hiebl J, Zhiral E. Liebigs Ann Chem. 1988:765. [Google Scholar]
- 4.Feuer H, Rubinstein H, Nielsen AT. J Org Chem. 1958;33:1107. [Google Scholar]
- 5.Br2-DMSO: Johnson HW, Jr, Daughheter PH., Jr J Org Chem. 1964;29:246.acid-DMSO: Martin D, Weise A. Angew Chem Int Ed. 1967;6:168.
- 6.Johnson HW, Jr, Krutzsch H. J Org Chem. 1967;32:1939. [Google Scholar]
- 7.(a) Ichikawa Y, Nishiyama T, Isobe M. J Org Chem. 2001;66:4200. doi: 10.1021/jo0100751. [DOI] [PubMed] [Google Scholar]; (b) Ichikawa Y, Ohara F, Kotsuki H, Nakane K. Org Lett. 2006;8:5009. doi: 10.1021/ol0616788. [DOI] [PubMed] [Google Scholar]
- 8.IR and NMR spectroscopic values matched published values.
- 9.Isocyanate 2d is reportedly difficult to purify: Alisi MA, Brufani M, Filocamo L, Gostoli G, Licandro E. Bioorg Med Chem Lett. 1995;5:2077.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.