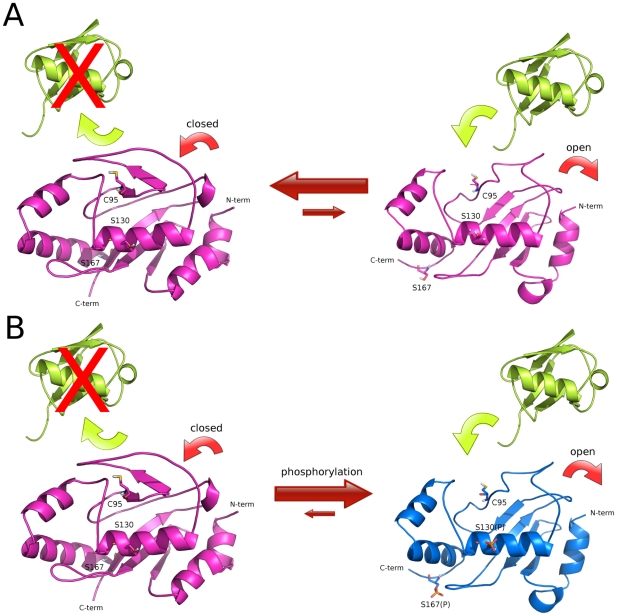
Figure 10. Model of the activation mechanism of ubiquitin charging activity of Cdc34-like E2 enzymes.
Ubiquitin, unphosphorylated and phosphorylated Cdc34-like E2s are shown in yellow, magenta and blue, respectively. (A) In unphosphorylated E2 enzyme, the acidic loop can interconvert between open and closed conformations with respect to the catalytic site and ubiquitin charging is strongly disadvantaged. (B) Upon phosphorylation, the E2 enzyme is stabilized in an open conformation of the acidic loop, competent for ubiquitin charging, induced by electrostatic repulsion, which do not allow the closure of the loop on the catalytic cleft.