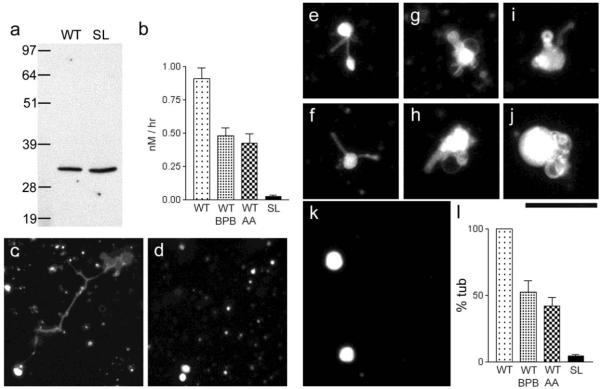
Figure 4. Phospholipase activity of MLN1 and evidence showing that the serine lipase site of MLN1 is required for the MLN1-mediated tubule formation from model membrane vesicles.
Panel (a) shows Western blots of the WT and SL fragments of MLN1 produced in vitro as described in Methods. TLC and the Bodipy FL C5 –HPC probe were used to measure the PLA2 activity of the WT and SL fragments of MLN1 and the reduction of this activity of WT-MLN1 by 40 μM BPB and aristolochic acid (AA), as shown in (b). In the experiments on the model membrane system the WT-MLN1 fragment mediated formation of tubulo-vesicular structures after treating liposomes that were prepared from Folch fraction I phospholipids stained with sulforhodamine-PE for 5 hr at 37°C (c), while no such structures were observed with the SL-MLN1 fragment (d). The WT-MLN1 fragment also generated tubules and vesicular enlargements from liposomes formed from asolectin phospholipids stained with NBD-PS (e-j). The SL-MLN1 fragment again failed to produce such structures in this model membrane system (k). Panel (l) summarizes the quantitative data from the experiments on the liposomes. The percentage of tubular structures per field generated under each of the different conditions was normalized in relation to that mediated by the WT-MLN1 fragment that was taken as 100%. The PLA2 inhibitors, BPB and aristolochic acid (AA), reduced significantly the number of tubular structures (+/−SEM, n=6). Scale bars are 50 μm.