Abstract
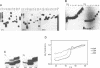
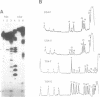
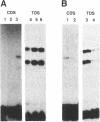
Unusual DNA structures involving four guanines in a planar formation (guanine tetrads) are formed by guanine-rich (G-rich) telomere DNA and other G-rich sequences (reviewed in (1)) and may be important in the structure and function of telomeres. These structures result from intrastrand and/or interstrand Hoogsteen base pairs between the guanines. We used the telomeric repeat of Chlamydomonas reinhardtii, TTTTAGGG, which contains 3 guanines and has a long interguanine A + T tract, to determine whether these sequences can form intrastrand and interstrand guanine tetrads. We have found that ss (TTTTAGGG)4 can form intrastrand guanine tetrads that are less stable than those formed by more G-rich telomere sequences. They are not only more stable, but also more compact, they are more stable in the presence of K+ than they are in the presence of Na+. While ds oligonucleotides with ss 3' overhangs of (TTTTAGGG)2 can be observed to associate as dimers, formation of this interstrand guanine tetrad structure occurs to a very limited extent and requires very high G-strand concentration, high ionic strength, and at least 49 hours of incubation. Our results suggest that, if telomere dimerization occurs in vivo, it would require factors in addition to the TTTTAGGG telomere sequence.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acevedo O. L., Dickinson L. A., Macke T. J., Thomas C. A., Jr The coherence of synthetic telomeres. Nucleic Acids Res. 1991 Jun 25;19(12):3409–3419. doi: 10.1093/nar/19.12.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agard D. A., Sedat J. W. Three-dimensional architecture of a polytene nucleus. Nature. 1983 Apr 21;302(5910):676–681. doi: 10.1038/302676a0. [DOI] [PubMed] [Google Scholar]
- GELLERT M., LIPSETT M. N., DAVIES D. R. Helix formation by guanylic acid. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guschlbauer W., Chantot J. F., Thiele D. Four-stranded nucleic acid structures 25 years later: from guanosine gels to telomer DNA. J Biomol Struct Dyn. 1990 Dec;8(3):491–511. doi: 10.1080/07391102.1990.10507825. [DOI] [PubMed] [Google Scholar]
- Hardin C. C., Henderson E., Watson T., Prosser J. K. Monovalent cation induced structural transitions in telomeric DNAs: G-DNA folding intermediates. Biochemistry. 1991 May 7;30(18):4460–4472. doi: 10.1021/bi00232a013. [DOI] [PubMed] [Google Scholar]
- Henderson E. R., Blackburn E. H. An overhanging 3' terminus is a conserved feature of telomeres. Mol Cell Biol. 1989 Jan;9(1):345–348. doi: 10.1128/mcb.9.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. R., Moore M., Malcolm B. A. Telomere G-strand structure and function analyzed by chemical protection, base analogue substitution, and utilization by telomerase in vitro. Biochemistry. 1990 Jan 23;29(3):732–737. doi: 10.1021/bi00455a020. [DOI] [PubMed] [Google Scholar]
- Henderson E., Hardin C. C., Walk S. K., Tinoco I., Jr, Blackburn E. H. Telomeric DNA oligonucleotides form novel intramolecular structures containing guanine-guanine base pairs. Cell. 1987 Dec 24;51(6):899–908. doi: 10.1016/0092-8674(87)90577-0. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Swanton M. T., Donini P., Prescott D. M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3' terminus. Proc Natl Acad Sci U S A. 1981 May;78(5):3015–3019. doi: 10.1073/pnas.78.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin S. Models of specifically paired like (homologous) nucleic acid structures. J Mol Biol. 1971 Jan 28;55(2):293–298. doi: 10.1016/0022-2836(71)90201-4. [DOI] [PubMed] [Google Scholar]
- Oka Y., Thomas C. A., Jr The cohering telomeres of Oxytricha. Nucleic Acids Res. 1987 Nov 11;15(21):8877–8898. doi: 10.1093/nar/15.21.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyutin I. G., Kovalsky O. I., Budowsky E. I., Dickerson R. E., Rikhirev M. E., Lipanov A. A. G-DNA: a twice-folded DNA structure adopted by single-stranded oligo(dG) and its implications for telomeres. Proc Natl Acad Sci U S A. 1990 Feb;87(3):867–870. doi: 10.1073/pnas.87.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracek M. E., Lefebvre P. A., Silflow C. D., Berman J. Chlamydomonas telomere sequences are A+T-rich but contain three consecutive G-C base pairs. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8222–8226. doi: 10.1073/pnas.87.21.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman M. K., Cech T. R. Effect of monovalent cation-induced telomeric DNA structure on the binding of Oxytricha telomeric protein. Nucleic Acids Res. 1990 Aug 11;18(15):4543–4552. doi: 10.1093/nar/18.15.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D., Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature. 1990 Mar 29;344(6265):410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988 Jul 28;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- Sundquist W. I., Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989 Dec 14;342(6251):825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- Thiele D., Guschlbauer W. Protonated polynucleotide structures. IX. Disproportionation of poly (G)-poly (C) in acid medium. Biopolymers. 1971;10(1):143–157. doi: 10.1002/bip.360100111. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Raghuraman M. K., Cech T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989 Dec 1;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Williamson J. R., Cech T. R., Prescott D. M. Inhibition of telomerase by G-quartet DNA structures. Nature. 1991 Apr 25;350(6320):718–720. doi: 10.1038/350718a0. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]