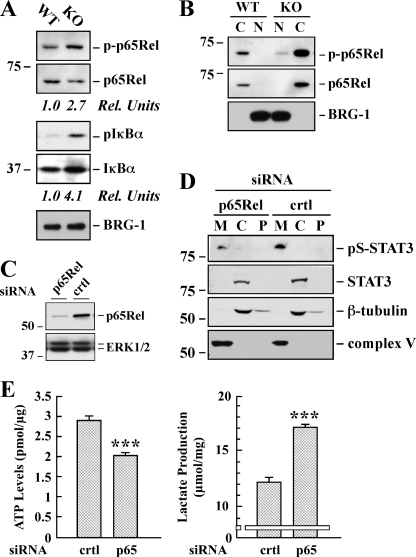
FIGURE 6.
Effect of p65Rel silencing on STAT3 expression and ATP/lactate ratio in SirT1-KO MEFs. A, total lysates of WT and Sirt1-KO MEFs were prepared and analyzed by Western blot for total and phosphorylated p65Rel, as well as total and phosphorylated IκBα. The blot was reprobed for BRG1 as a loading control. Rel Units, for each phospho-specific antibody, a phosphorylation signal ratio change (phospho/total) was calculated and normalized to the WT sample. The migration of molecular-mass markers (values in kilodaltons) is shown on the left of immunoblots. B, cytosolic (C) and nuclear (N) fractions were prepared from the WT and Sirt1-KO MEFs and processed for immunoblot analysis. The blot was reprobed with anti-BRG1 antibody to demonstrate the quality of our nuclear fractionation. C, Sirt1-KO MEFs were transfected with 40 nm of either control (crtl) or a pool of four siRNAs targeting p65Rel. Following 48 h of siRNA knockdown, cell lysates were subjected to Western blot analysis for p65Rel and ERK1/2 expression levels. D, mitochondrial (M), cytosolic (C), and P100 membrane (P) fractions were prepared from Sirt1-null MEFs transfected with either control or p65-Rel siRNA. Western blot analysis was performed with the indicated antibodies. Similar results were obtained in two independent experiments performed in triplicate dishes. Ser(P)-STAT3 (pS-STAT3). E, control and p65Rel-depleted Sirt1-KO cells were processed for lactate determination and ATP levels. The data represents the average ± S.D. of three independent experiments. ***, p < 0.001 versus control siRNA-transfected cells.