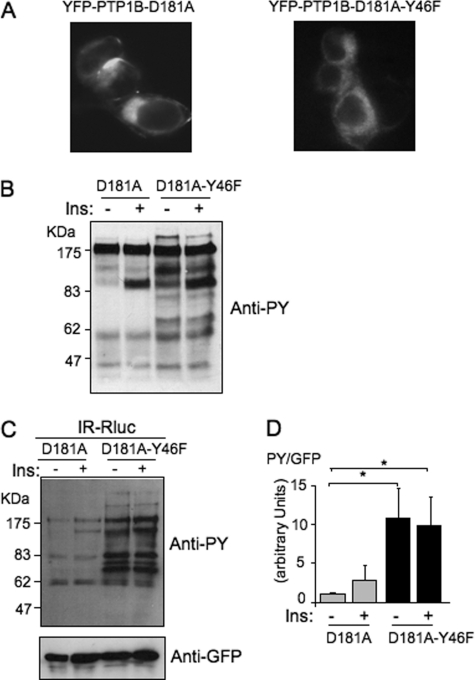
FIGURE 1.
The PTP1B-D181A-Y46F mutant is a much more efficient substrate-trapping mutant than PTP1B-D181A. A, localization of the YFP-tagged mutants of PTP1B was observed by fluorescent microscopy. B, HEK-293 cells transfected with the IR and either YFP-PTP1B-D181A or YFP-PTP1B-D181A-Y46F were incubated for 5 min in the absence (−) or presence (+) of 100 nm insulin. 50 μg of proteins was loaded on a gel and submitted to Western blotting using an anti-phosphotyrosine antibody (4G10). C, HEK-293 cells transfected with IR-Rluc and either YFP-PTP1B-D181A or YFP-PTP1B-D181A-Y46F were stimulated for 5 min with 100 nm insulin (Ins). Cells were extracted, and the YFP fluorescence was evaluated on an aliquot of the extract by measuring light emission at 530 nm after illumination at 485 nm. Equivalent amounts of fluorescent PTP1B were then immunoprecipitated using an anti-GFP antibody. Tyrosine phosphorylation of proteins coimmunoprecipitated with PTP1B was evaluated by immunoblotting using an anti-phosphotyrosine antibody (4G10). The amount of PTP1B loaded in each lane was then controlled by reprobing the membrane using an anti-GFP antibody. D, densitometric analysis of the total tyrosine phosphorylation level of immunoprecipitated proteins. The signal corresponding to the total level of tyrosine-phosphorylated proteins detected in each lane was corrected by the amount of PTP1B in the same lane as evaluated by using the anti-GFP antibody. Results are the mean ± S.E. of four independent experiments. *, p < 0.05 when compared with the unstimulated D181A control condition using analysis of variance followed by Dunnett's multiple comparison test.