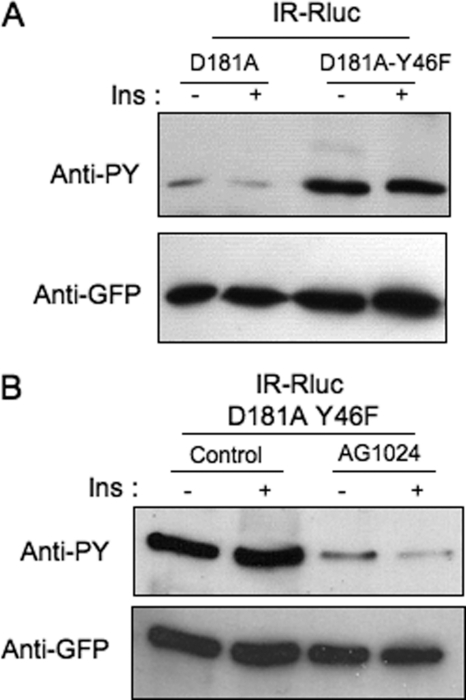
FIGURE 2.
Hyperphosphorylation of the PTP1B-D181A-Y46F mutant on tyrosine residues by the insulin receptor. A, HEK-293 cells transfected with IR-Rluc and either YFP-PTP1B-D181A or YFP-PTP1B-D181AY46F were incubated for 5 min in the absence (−) or presence (+) of 100 nm insulin (Ins). Proteins were extracted, and PTP1B was immunoprecipitated using an anti-GFP antibody. Tyrosine phosphorylation of PTP1B was evaluated by immunoblotting using an anti-phosphotyrosine antibody (4G10). The amount of PTP1B loaded in each lane was evaluated by reprobing the membrane using an anti-GFP antibody. Results are representative of five independent experiments. B, HEK-293 cells transfected with IR-Rluc and YFP-PTP1B-D181A-Y46F were preincubated for 1 h in the absence or presence of 100 μm AG1024 and incubated with 100 nm insulin for 5 min. Cells were then lysed, and PTP1B was immunoprecipitated and analyzed by Western blotting using an anti-phosphotyrosine antibody (4G10). The amount of PTP1B loaded in each lane was controlled using an anti-GFP antibody. Results are representative of three independent experiments.