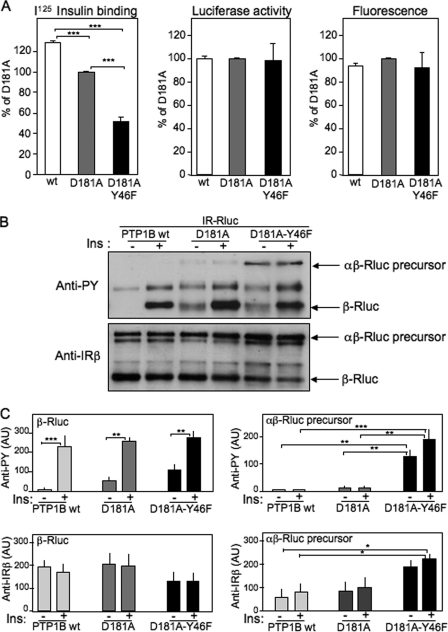
FIGURE 5.
Effect of the PTP1B-D181A-Y46F mutant on cell surface expression of the insulin receptor. A, cell surface I125 insulin binding was measured 48 h after transfection of HEK-293 cells with IR-Rluc and YFP-PTP1B-WT, YFP-PTP1B-D181A, or YFP-PTP1B-D181A-Y46F. Luciferase activity and YFP fluorescence levels were measured in parallel for evaluation of total IR-Rluc and YFP-PTP1B expression on the same cells. Results are the mean ± S.E. of four to nine independent experiments. B, 48 h after transfection, cells were incubated in absence (−) or presence (+) of 100 nm insulin (Ins) for 5 min. After cell extraction, the IR was partially purified on wheat germ lectin-agarose beads and submitted to Western blotting. The tyrosine phosphorylation level of the receptor was evaluated using an anti-phosphotyrosine antibody (4G10). The amounts of uncleaved IR precursor (αβ-Rluc) and mature cleaved receptor (β-Rluc) were evaluated using an anti-receptor β-subunit antibody. C, densitometric analysis of the autoradiograms. Results are the mean ± S.E. of five independent experiments. AU, arbitrary units. *, p < 0.05; **, p < 0.01; ***, p < 0.001 using analysis of variance followed by Tukeys's multiple comparison test.