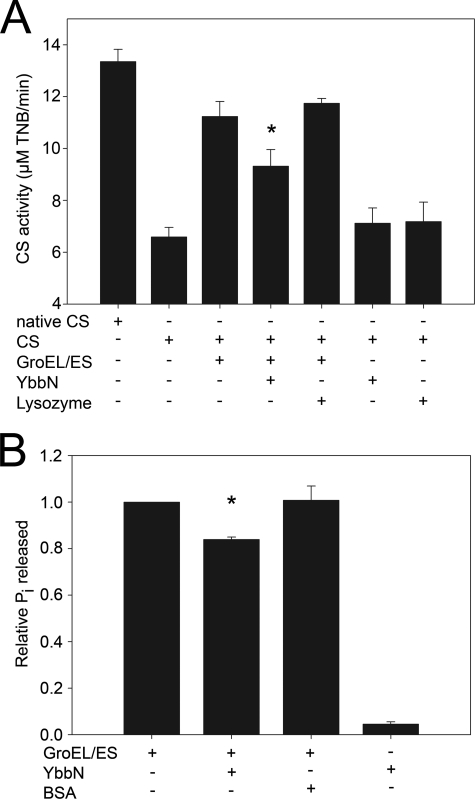
FIGURE 7.
GroESL activity is mildly inhibited by YbbN. Panel A shows the amount of CS activity recovered from chemically denatured enzyme that was refolded by dilution into buffer containing the proteins indicated by a + sign in the table at the bottom of the graph. The reaction was followed by monitoring the production of colored 2-nitro-5-thiobenzoate by liberated CoA. Native CS, which had not been denatured before the assay, is the positive control. The GroESL chaperonin leads to a substantial recovery of CS activity that is diminished by the addition of YbbN. The asterisk indicates that the difference between values with and without YbbN is significant to a p value <0.05 by Student's t test for data generated from three independent experiments. YbbN alone is no more effective than the negative control protein lysozyme at refolding CS. Panel B shows the release of inorganic phosphate from ATP by the GroEL ATPase activity as determined using the Malachite Green assay. Samples containing the protein(s) are indicated by a + sign in the table at the bottom of the graph. The addition of YbbN results in a statistically significant (asterisk; p value < 0.05 by Student's t test) decrease in the GroEL ATPase activity, in agreement with the diminution of chaperone activity shown in panel A. Bovine serum albumin (BSA) was used as a negative control. Data are from three independent experiments.