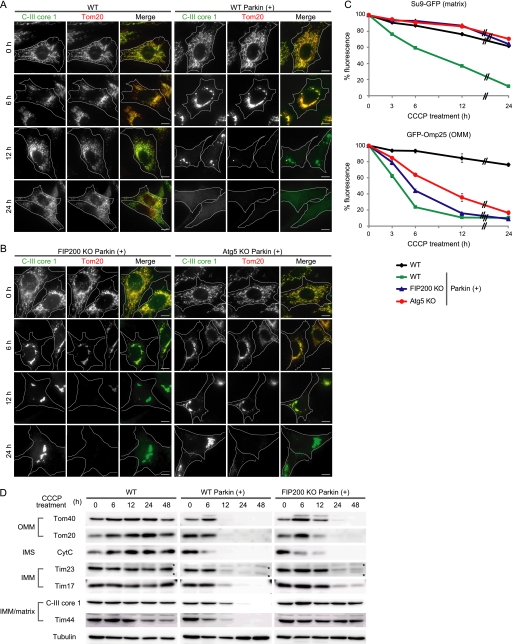
FIGURE 1.
OMM proteins are degraded mainly in an autophagy-independent manner in depolarized mitochondria. A and B, wild-type (WT) MEFs with and without stable Parkin expression (A) and FIP200 KO and Atg5 KO MEFs with Parkin expression (B) were treated with 20 μm CCCP for different time periods as indicated. Cells were immunostained for C-III core 1 (an IMM/matrix protein) and Tom20 (an OMM protein). Signal color is indicated by the colored text. Scale bars, 10 μm. C, wild-type MEFs (with and without exogenous Parkin expression) and FIP200 KO and Atg5 KO MEFs (with exogenous Parkin expression) expressing either Su9-GFP (a matrix protein) or GFP-Omp25 (an OMM protein) were treated with 20 μm CCCP for different time periods as indicated. The GFP fluorescence was quantified by flow cytometry. Data represent mean ± S.E. of three independent experiments. D, wild-type MEFs (with and without exogenous Parkin expression) and FIP200 KO MEFs (with exogenous Parkin expression) were treated with 20 μm CCCP for different time periods as indicated. The cells were analyzed by SDS-PAGE and subsequent immunoblotting with antibodies against Tom40 and Tom20 (OMM proteins), cytochrome c (CytC) (an intermembrane space (IMS) protein), Tim23 and Tim17 (IMM proteins), and C-III core 1 and Tim44 (IMM/matrix proteins), as well as α-tubulin (a loading control). Asterisks indicate nonspecific immunoreactive bands.