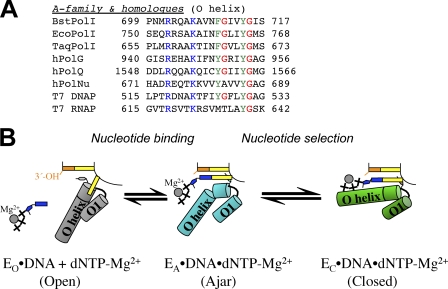
FIGURE 5.
A, shown is structure-based manual sequence alignment of nucleotide binding helices of A family DNA polymerases. Blue, phosphate interaction; green, aromatic residues in the nucleotide binding sites; red, proposed glycine hinges in the helices. BstPolI, EcoPolI, and TaqPolI, DNA polymerase I from B. stearothermophilus, E. coli, and Thermus aquaticus; hPolG, hPolQ, and hPolNu, human DNA polymerases γ, θ, and ν. B, a model for nucleotide sampling and selection is shown. Nucleotides are sampled in the ajar conformation (EA, cyan) and are released if it incorrect, whereupon the enzyme returns to the open conformation (EO, gray) or entrapped in the closed conformation (EC, green) if it is complementary to the template base. Schematic representations of each state are shown in the center.