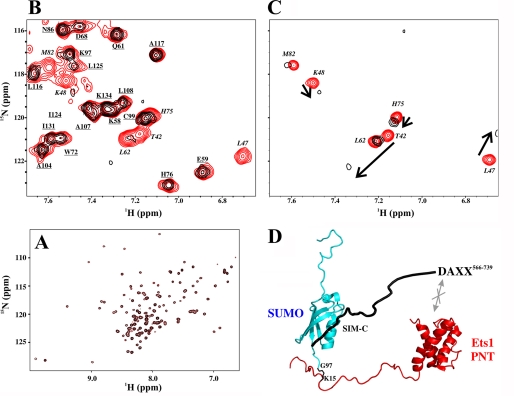
FIGURE 7.
SIM-C of DAXX566–739 binds only the SUMO-1 moiety of sumoylated Ets1. A, superimposed 15N HSQC spectra of sumoylated 15N-labeled Ets11–138 in the absence (red) and presence (black) of unlabeled DAXX566–739. The lack of any detectable shift or intensity perturbations in the amide signals of Ets11–138 demonstrates that the Ets1 and DAXX fragments did not interact directly with any appreciable affinity. Note that the SUMO moiety of this covalently linked species was unlabeled and hence invisible. B, superimposed 15N HSQC spectra of a 15N-labeled SUMO-11–97-Ets116–138 chimera in the absence (290 μm; red) and presence (black) of 1.9 eq of unlabeled DAXX566–739. Only residues from the SUMO-1 moiety (italicized) showed severe signal broadening due to the higher molecular mass of the resulting complex. In contrast, peaks arising from amides in Ets116–138 (underlined) were unperturbed. C, superimposed 15N HSQC spectra of 15N-labeled SUMO-1 in the absence (120 μm; red) and presence (black) of 9 eq of unlabeled DAXX566–739. The same residues as those that disappeared in the spectrum of the chimera showed intermediate exchange broadening, thus confirming that SUMO-1 functions as an independent tag on Ets1. D, beads-on-a-string model for the non-covalent binding of DAXX566–739 via SIM-C to sumoylated Ets11–138, emphasizing the lack of any direct interaction between the PNT domain-containing fragment of Ets1 and the intrinsically disordered C-terminal segment of DAXX.