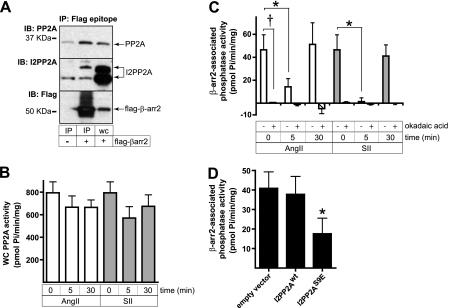
FIGURE 4.
Transient inhibition of β-arrestin 2-bound PP2A in response to SII and AngII treatment. A, coimmunoprecipitation (IP) of the endogenous PP2A catalytic subunit and I2PP2A with FLAG-tagged β-arrestin 2. HEK-AT1AR cells were transiently transfected with either empty vector or FLAG-β-arrestin 2 and FLAG immunoprecipitates performed as described. The presence of coprecipitating PP2A and I2PP2A was demonstrated by immunoblotting (IB). A whole cell lysate lane (WC) is shown for comparison. B, effect of SII and AngII treatment on whole cell PP2A activity. HEK-AT1AR cells were stimulated with SII or AngII for the indicated times, after which the PP2A catalytic subunit was immunoprecipitated and PP2A activity determined as described. Neither ligand produced a significant change in global PP2A activity (n = 5). C, transient inhibition of FLAG-β-arrestin 2-bound PP2A following SII or AngII stimulation. HEK-AT1AR cells transiently expressing FLAG-tagged β-arrestin 2 were stimulated for the indicated times with SII or AngII, after which FLAG-β-arrestin 2-associated Ser phosphatase activity was determined as described. Background phosphatase activity (average 24 pmol Pi/min/mg protein) was measured in FLAG immunoprecipitates performed on vector-transfected control cells and subtracted from that measured in FLAG-β-arrestin 2 immunoprecipitates. Parallel samples were incubated for 10 min with okadaic acid (2 nm) after immunoprecipitation to determine the extent to which PP2A contributed to FLAG-β-arrestin 2-bound phosphatase activity. Data shown are the mean ± S.E. of FLAG-β-arrestin 2-bound phosphatase activity determined under each condition. *, t test p < 0.05, less than NS (n = 5). †, p < 0.05, okadaic acid treated less than untreated (n = 5). D, inhibition of FLAG-β-arrestin 2-bound phosphatase activity by the phosphomimetic I2PP2A-S9E mutant. HEK-AT1AR cells were transiently transfected with plasmids encoding FLAG-tagged β-arrestin 2 and either empty vector, wild type I2PP2A, or I2PP2A-S9E. FLAG immunoprecipitates were isolated, and β-arrestin 2-associated PP2A activity was determined as described. The bar graph depicts mean ± S.E. of phosphatase activity measured under each condition. *, t test p < 0.05, less than NS (n = 3).