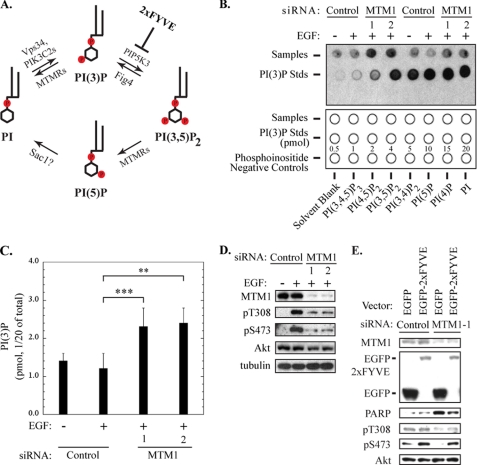
FIGURE 4.
Increased PI(3)P levels are linked to inhibition of Akt phosphorylation and pro-apoptotic signaling in myotubularin-deficient HeLa cells. A, routes for cyclic interconversion and regulation of the MTMR-specific phosphoinositides PI(3)P and PI(3,5)P2 are depicted. Phosphoinositide kinases associated with specific steps in this pathway (PIK3C2A, PIK3C2B, Vps34, PIP5K3) are listed above the arrows, and regulatory points for myotubularin family phosphatases (MTMRs) and other phosphatases that regulate these inositol lipids (Sac1) are shown below the arrows. The point at which the EGFP-2xFYVE PI(3)P-specific binding protein interferes with conversion of PI(3)P to PI(3,5)P2 is shown at the upper right. B, PI(3)P levels in control or myotubularin-deficient HeLa cells were quantified using a PI(3)P mass assay kit according to the manufacturer's protocol. An image of the developed film representing two independent sample sets and the PI(3)P standard curve is shown in the upper panel. The lower panel indicates the locations of HeLa samples (1/20 of total sample) (top row), PI(3)P standards (center row), and phosphoinositide negative control lipids (bottom row) that were spotted on the membrane for analysis. C, spots corresponding to PI(3)P derived from HeLa cell experimental samples and the PI(3)P standards on developed film were quantified by scanning densitometry. PI(3P levels from control and myotubularin-deficient HeLa samples were determined by comparison to PI(3)P standards and are represented graphically (mean ± S.D., n = 3; **, p < 0.01; ***, p < 0.005). D, soluble protein extracts from HeLa cells prepared in parallel with those used for PI(3)P quantification were analyzed by immunoblotting for myotubularin (MTM1), phosphorylated Akt (Thr(P)-308 (pT308), Ser(P)-473 (pS473)), and total Akt. Tubulin immunoblotting was used as a loading control. E, HeLa cells were transfected with EGFP or EGFP-2xFYVE expression vectors for 24 h, then transfected with control siRNA or myotubularin siRNA-1 for an additional 48 h. Before harvest, the cells were serum-deprived and stimulated with insulin. Soluble protein extracts were analyzed by immunoblotting for the indicated proteins.