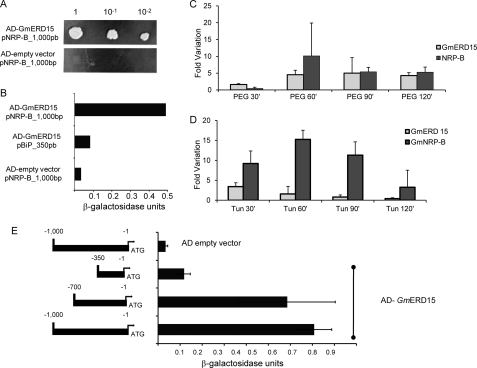
FIGURE 1.
A tunicamycin- and PEG-induced ERD15 soybean homolog associates stably with the NRP-B promoter in yeast. A, isolation of GmERD15 by one-hybrid screening in yeast. An S. cerevisiae strain carrying the pNRPB-HIS3 reporter was transformed with the GAL4 activation domain-encoded vector or GAL4AD vector containing the cDNA of GmERD15. After growth for 12 h, the yeast culture was diluted, as indicated in the figure, and plated on medium lacking histidine but supplemented with 100 mm 3-aminotriazole and incubated for 3 days. B, LacZ reporter expression. β-Galactosidase activity was determined from total protein extracts of yeast strains carrying the indicated combinations of the empty expression vector and AD-GmERD15. As a control, a pBip_350bp-LacZ integrated strain was transformed with AD-GmERD15. Thin bars indicate the standard deviation of three independent experiments. C, kinetics of GmERD15 and GmNRP-B induction by PEG-induced osmotic stress in soybean-cultured cells. Levels of RNA were examined at various times after PEG treatment by quantitative RT-PCR. Expression values were calculated using the 2 − ΔCt method with helicase used as an endogenous control. cDNA was prepared from three biological replicates. Thin bars indicate the standard deviation. D, induction of GmERD15 and NRP-B by tunicamycin. Soybean-cultured cells were treated with tunicamycin for the indicated times. Levels of RNA were examined by quantitative RT-PCR, as described in C. E, GmERD15 associates to a discrete region of NRP-B promoter in yeast. The schematic representation of the promoter constructs indicates the 5′-flanking sequences of NRP-B fused to LacZ that were integrated into the W303 strain and transformed with AD-GmERD15 or the empty vector. The activity of the β-galactosidase was expressed in units as described under “Experimental Procedures.” Thin bars indicate the standard deviation of three independent experiments.