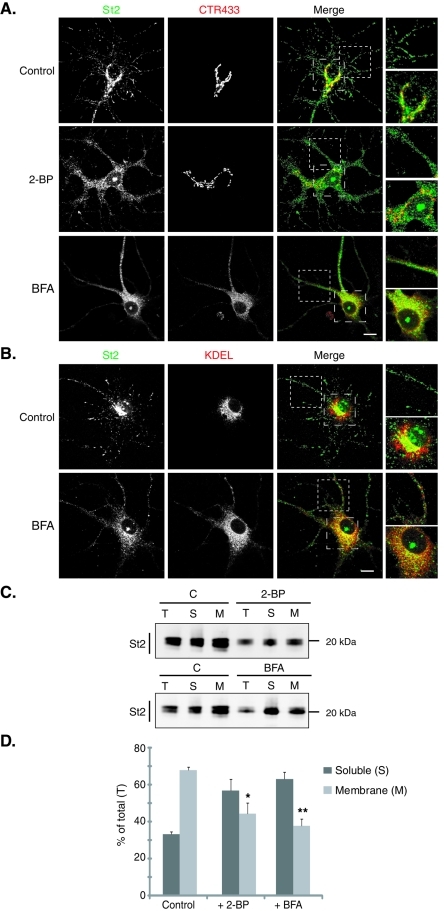
FIGURE 2:
Preventing palmitoylation leads to the dispersion of the Golgi pool of stathmin 2. Hippocampal neurons at 6 DIV were stained for stathmin 2 (St2, green) and for either the Golgi (CTR433, red) or the ER (KDEL, red). For each double labeling, a single-layer confocal image is shown. (A) In untreated neurons (control), stathmin 2 immunolabeling is present at the Golgi (colocalization with CTR433) and along neurites as vesicle-like structures. Treatments with 2-BP and BFA resulted in the dispersion of the Golgi pool of stathmin 2, whereas the vesicle-like localization was partially abolished. Higher magnification of the boxed neurite and soma regions in merged images better illustrates the dispersion of the Golgi stathmin 2 labeling as well as the small size of the remaining vesicle-like structures present after both treatments. (B) Following BFA treatment, stathmin 2 was not relocalized to the ER, neither in the soma nor within neurites (see higher magnification of the merged images). Bars = 10 μm. (C) The subcellular localization of stathmin 2 was analyzed after cell fractionation that separates the soluble (S) and membrane (M) fractions from total extracts (T) of untreated (C), 2-BP, or BFA-treated hippocampal neurons. Equivalent volumes of each fraction were analyzed by SDS–PAGE and Western blotted for stathmin 2. (D) Quantification of the membrane and soluble pools of stathmin 2 expressed as a percentile of stathmin 2 in the total extract. Whereas stathmin 2 was present in the majority of the membrane fraction in the control, it was significantly redistributed from the membrane to the soluble fraction after 2-BP or BFA treatment. Values are mean ± SEM (n = 3). The statistical significance of differences between stathmin 2 in membrane fractions when treated or not by 2-BP or BFA was assessed by a Student's t test. *p < 0.05, **p < 0.01.