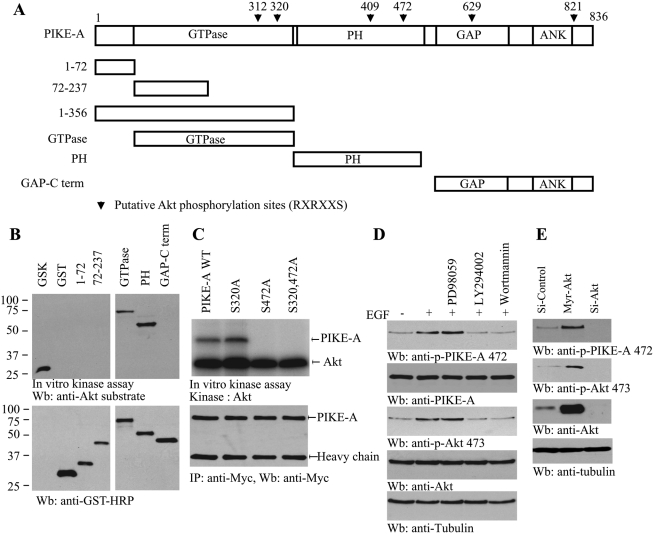
FIGURE 1:
Akt phosphorylates PIKE-A on Ser-472. (A) The diagram of human PIKE-A. PIKE-A possesses six putative Akt phosphorylation motifs (RXRXX(S/T)), as indicated (▾) with residue numbers. (B) In vitro Akt kinase assay. Purified recombinant GST-fusion proteins were incubated with active Akt at 30°C for 30 min. GTPase and PH domains were robustly phosphorylated, while other fragments were not. GSK was a positive control. (C) Ser-472 residue in PIKE-A is phosphorylated by Akt. Myc–PIKE-A wild-type, Ser-320A, and Ser-472A and Ser-320/Ser-472A were incubated with active Akt at 30°C for 30 min. Wild-type PIKE-A and Ser-320A but not Ser-472A mutant were strongly phosphorylated (top). Equal amounts of immunoprecipitated proteins were employed (bottom). (D) PI 3-kinase inhibitors block PIKE-A phosphorylation on Ser-472. Brain cells were pretreated by 10 μM LY294002, 100 nM wortmannin, or 10 μM PD98059 and then treated with growth factor for 20 min. EGF stimulated PIKE-A phosphorylation, which was diminished by PI 3-kinase but not ERK inhibitor pretreatment (top left). Akt phosphorylation was verified (left, second panel). (E) Akt is required for PIKE-A Ser-472 phosphorylation. Active Akt robustly phosphorylates endogenous PIKE-A and knocking down of Akt eliminates its phosphorylation (top). Verification of Akt overexpression and knockdown (third panel).