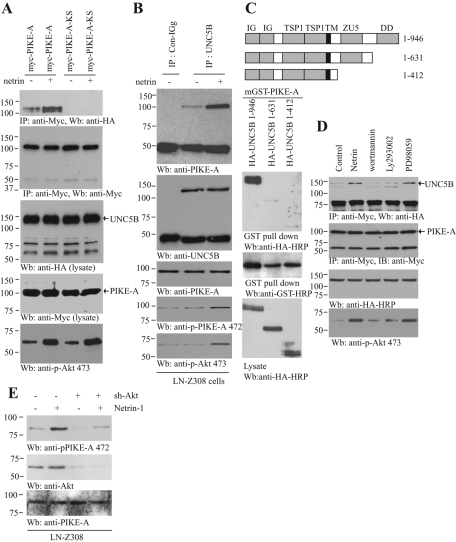
FIGURE 2:
Netrin-1–activated Akt mediates interaction between PIKE-A and UNC5B. (A) Netrin-1 increases the binding of PIKE-A to UNC5B. Wild-type and GTPase-dead (KS) PIKE-A were cotransfected into HEK293 cells with HA-UNC5B. The transfected cells were treated with netrin-1 for 30 min. PIKE-A was immunoprecipitated with anti-Myc antibody and analyzed with anti-HA antibody. Equal amounts of HA-UNC5B and Myc-PIKE-A were immunoprecipitated (second and third panels). (B) PIKE-A interaction with UNC5B in LN-Z308 cells. LN-Z308 cells were treated with netrin-1 for 30 min, endogenous UNC5B was immunoprecipitated with control IgG or anti-UNC5B antibody and the bound PIKE-A was detected using anti–PIKE-A antibody (top). Confirmation of Akt and PIKE-A phosphorylation status (fourth and fifth panels). (C) The UNC5B death domain is essential for the interaction between UNC5B and PIKE-A. The diagram of UNC5B domains is shown (top). Different UNC5B truncates were cotransfected into HEK293 cells with mGST–PIKE-A. PIKE-A was pulled down with glutathione beads and analyzed with anti-HA antibody. Truncation of the death domain in UNC5B or its whole intracellular domain abolished the interaction between PIKE-A and UNC5B (bottom). (D) PI 3-kinase signaling regulates the interaction between PIKE-A and UNC5B. HA-UNC5B and Myc–PIKE-A cotransfected HEK293 cells were pretreated with PD98059 (10 μM), wortmannin (100 nM), LY294002 (10 μM) for 30 min, before netrin-1 was introduced. Myc–PIKE-A was immunoprecipitated using Myc antibody, and the bound HA-UNC5B was detected using anti–HA-HRP antibody. Netrin-1 triggered the interaction between PIKE-A and UNC5B, but PI 3-kinase inhibitor markedly blocked it (top). Confirmation of Akt phosphorylation status (bottom). (E) Netrin-triggered PIKE-A phosphorylation is Akt dependent. LN-Z308 cells were infected with ad-shRNA-Akt for 36 h followed by 200 ng/ml netrin-1 treatment for 30 min. Immunoblotting was conducted with anti–phospho-PIKE-A Ser-472 (top), anti-Akt (middle), and anti–PIKE-A (bottom) antibodies.