Scheme 3.
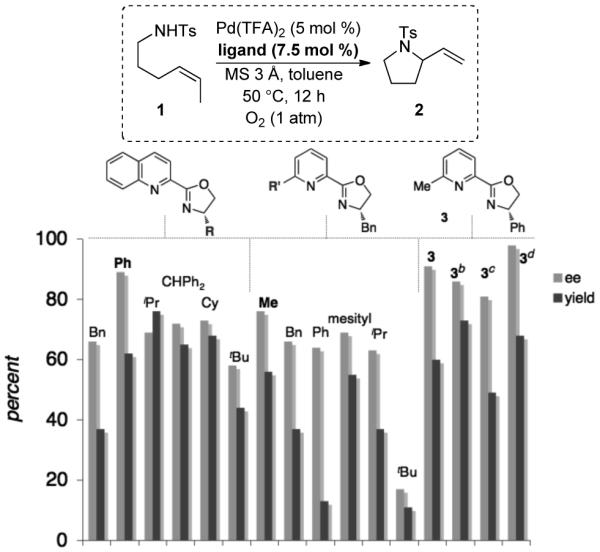
Ligand Substituent Effects and Reaction Optimization.a
a Conditions: 1 (0.075 mmol), 1 atm O2, MS 3 Å (20 mg), toluene (0.75 mL), 12 h. Yield determined by 1H NMR spectroscopy, internal standard = 1,3,5-trimethoxybenzene. Enantiomeric excess deteremined by chiral HPLC (see Supporting Information for details). b 5.5 mol % ligand. c Without MS 3 Å. d 25 °C, 24 h. Isolated yield (0.5 mmol scale).
