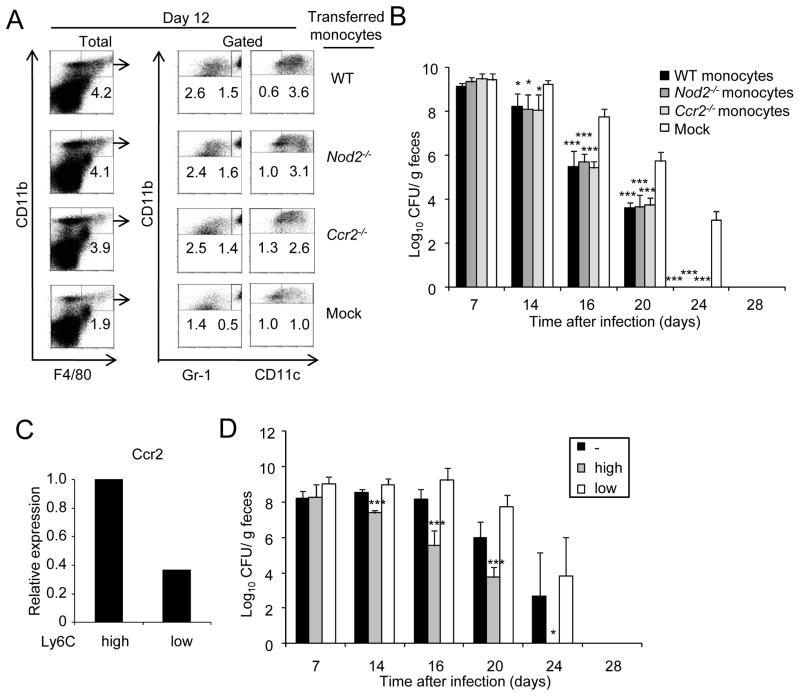
Figure 7. Ly6Chi but not Ly6Clo monocytes promote clearance of C. rodentium.
(A) Bone marrow monocytes from WT, Nod2−/−, and Ccr2−/− mice were adoptively transferred into Ccr2−/− mice previously challenged orally with C. rodentium. Four days after oral infection with C. rodentium, mice received the cells or received PBS (mock) by i.v. injection. Cells were isolated from the colon of WT monocytes → Ccr2−/− mice, Nod2−/− monocytes → Ccr2−/− mice, Ccr2−/− monocytes → Ccr2−/− mice or received no monocytes (mock) on day 0, and 12 after oral infection with C. rodentium. Intestinal cells were stained for CD11b, F4/80, and Gr-1 or CD11c. CD11b+F4/80+ double-positive cells (% of total cells, left panel) were gated and percentage of CD11b+F4/80+Gr-1lo, CD11b+F4/80+Gr-1hi, CD11b+F4/80+CD11clo, and CD11b+F4/80+CD11chi cells in the gated population was determined. Results are representative of at least two experiments. (B) C. rodentium numbers in stool from infected WT monocytes → Ccr2−/− mice, Nod2−/− monocytes → Ccr2−/− mice, Ccr2−/− monocytes → Ccr2−/− mice or mock → Ccr2−/− mice were determined by CFU assay. (C) Expression of Ccr2 in bone marrow Ly6Chi or Ly6Clo monocytes by real-time RT-PCR. mRNA expression of Ccr2 was normalized to that of β-actin. (D) C. rodentium numbers in stool from infected Ly6Chi monocytes → Ccr2−/− mice, Ly6Clo monocytes → Ccr2−/− mice, or mock → Ccr2−/− mice were determined by CFU assay (n= 5 mice per group). Data are means ± SD. Results are representative of 2 independent experiments.