Abstract
Aromatase inhibitors (AIs) have become the standard adjuvant therapy of postmenopausal breast cancer survivors. AIs induce a reduction of bioavailable estrogens by inhibiting aromatase, which would be expected to induce alterations in body composition, more extensive than induced by menopause. The objectives are to examine the impact of AIs on (1) DXA-scan derived body composition and (2) gonadal hormone levels. This is a sub-analysis of a 2-year double-blind, placebo-controlled, randomized trial of 82 women with nonmetastatic breast cancer, newly menopausal following chemotherapy, who were randomized to risedronate (35 mg once weekly) versus placebo, and stratified for their usage of AI versus no AI. Outcomes included DXA-scan derived body composition and gonadal hormone levels. As a group, total body mass increased in women over 24 months. Women on AIs gained a significant amount of lean body mass compared to baseline as well as to no-AI users (P < 0.05). Women not on an AI gained total body fat compared to baseline and AI users (P < 0.05). Free testosterone significantly increased and sex hormone binding globulin (SHBG) significantly decreased in women on AIs compared to no AIs at 24 months (P < 0.01) while total estradiol and testosterone levels remained stable. Independent of AI usage, chemotherapy-induced postmenopausal breast cancer patients demonstrated an increase of total body mass. AI users demonstrated maintenance of total body fat, an increase in lean body mass and free testosterone levels, and a decrease in SHBG levels compared to no-AI users. The mechanisms and implications of these changes need to be studied further.
Keywords: Breast cancer, Body composition, Gonadal hormones, Aromatase inhibitors
Background
There are over 100,000 new cases of hormone–receptor positive breast cancer per year in the US [1]. In order to reduce the risk of recurrence in postmenopausal women with hormone–receptor positive breast cancer, therapy with aromatase inhibitors [2] [AIs: anastrazole (Arimidex®), exemestane (Aromasin®), and letrozole (Femara®)], is currently recommended for 5 years and has become the standard of care due to their superior reduction of breast cancer recurrence compared to estrogen–receptor–agonists–antagonists [ERAAs, e.g., nolvadex (Tamoxifen®)] [3]. When a long-term, adjuvant treatment is recommended for early stage breast cancer to decrease the risk for distant relapse or new second primary breast cancer, patterns of adverse effects weigh heavily into treatment planning and monitoring. In postmenopausal women, the main source of estrogen is peripheral conversion from androgens by aromatase [2]. Since AIs inhibit aromatase, postmenopausal women taking AIs experience a further reduction in circulating, bio-available estrogens as well as a theoretical increase in androgens [2]. A prolonged period of profound estrogen suppression and (relative) hyperandrogenemia could be expected to induce major alterations in body composition and cardiovascular risk, possibly more extensive than those induced by menopause [4]. The effects of AIs on non-cancer outcomes and other aspects of the hormonal milieu have been under-explored.
The Risedronate’s Effect on Bone loss in Breast CAncer Study (REBBeCA Study) demonstrated that administration of risedronate once weekly for 2 years in newly postmenopausal women with breast cancer, positively affected spine and hip BMD and bone turnover, independent of their concurrent use of an aromatase inhibitor (AI) [5]. The objectives of this secondary analysis were to examine the impact of AIs versus no AIs (i.e., ERAAs and no hormonal therapy) on (1) body composition, using the whole body DXA-scan methodology (Hologic), and (2) gonadal hormone levels in these postmenopausal women.
Methods
Materials and methods
Study design
This secondary analysis reports the body composition and gonadal hormone changes of 82 of the 87 women in the parent study for whom adjuvant cancer therapy data were available, who had been enrolled in a 2-year double-blinded trial based on their recent postmenopausal status (≤8 years) after having received polyadjuvant chemotherapy for nonmetastatic breast cancer and who had body composition data available. Women had been randomized to active treatment with risedronate (n = 43, 35 mg po once weekly) or a matched placebo (n = 44), as previously described [5]. Briefly, women were not eligible if they had been diagnosed with a second primary cancer, abnormal bone, and mineral metabolism (due to medications or underlying disorders such as hyperthyroidism, malabsorption, renal failure, and hepatic failure), or current fracture.
The trial did not restrict concurrent use of adjuvant hormonal therapy as prescribed by their oncologist, allowing them to freely initiate or change a hormonal agent if medically necessary (e.g., AIs, ERAAs). Subjects were assigned to the AI group if they had been on anastrazole (Arimidex®), exemestane (Aromasin®), or letrozole (Femara®) for at least 3 months immediately preceding a follow-up assessment. Those who were not on adjuvant therapy or were on adjuvant hormonal therapy other than AIs [such as tamoxifen (Nolvadex®), toremifene (Fareston®), and fulvestrant (Faslodex®)] were assigned to the no-AI group.
The University of Pittsburgh Institutional Review Board approved the protocol. Participants were advised about the nature of the study and they provided written informed consent before participation.
Outcome variables
Body composition (total body mass, body fat, and lean body mass) was measured at each visit (0, 6, 12, 18, and 24 months) using body composition software on a Hologic QDR-4500A bone densitometer (Hologic Inc., Bedford, MA). The coefficients of variation for total fat mass is 2.1%, total lean mass 1%, and total fat is 1% [6].
Gonadal hormones [testosterone (total and free), estradiol (total), and sex hormone binding globulin (SHBG)] were measured via Radioimmunoassay (RIA) methodology by the Clinical and Translational Research Center of Johns Hopkins’s University. The corresponding inter- and intra-assay coefficients of variation are 5.39 and 4.73 for total testosterone, 5.67 and 5.00 for free testosterone, 6.97 and 3.72 for estradiol, and 6.54 and 6.54 for SHBG.
Statistical analysis
All data analysis was performed using SAS® version 9.2 (SAS Institute, Inc., Cary, North Carolina) with risedronate and placebo arms combined for greater statistical power in the absence of evidence that risedronate affects body composition and gonadal hormone levels. First, we compared the baseline characteristics of those on and not on an AI using independent samples t-tests. Next, for the analysis of body composition measures (collected every 6 months) analysis, we operationally defined a subject to have been an AI user at a given follow-up assessment if she had been on an AI for at least 3 of the 6 months since the previous assessment. In an initial model, we excluded from analysis any assessments of a subject after switching adjuvant cancer therapy from an AI to a no AI (or vice versa), and fit a linear mixed model with change from baseline in each body composition measure as the dependent variable; AI (yes/no), follow-up time (6, 12, 18, and 24 months) and their interaction as fixed effects of interest; and a subject random effect to obtain preliminary effects of AI on body composition. Next, these preliminary estimates were used to adjust body composition measures of those switching cancer therapy from a no AI to an AI in the middle of the study so that body compositions of those subjects could be analyzed as if they had been on AI from the start of the study. We used appropriately constructed contrasts to assess the AI effect at 6, 12, 18, and at 24 months. This process of estimation of effects and adjusting of outcomes was repeated a large number of times until the estimates stabilized and converged. This methodology has been successfully used elsewhere to address similar analyses [5]. For gonadal hormone analysis, we took a similar approach as above for the initial but did not perform the repetitive estimation process because data was collected only annually. Finally, we used Pearson product-moment correlations to examine associations between changes in gonadal hormones and changes in body composition. Both raw and percent changes were considered in all analyses to ensure robustness of results.
Findings
Clinical characteristics at baseline
Information about screening, randomization and follow-up are published in detail elsewhere [5]. At baseline, 11 women reported to consume an AI, 21 no hormonal therapy, while the remaining 50 women were on alternate hormonal therapy [of whom 47 nolvadex (Tamoxifen®)]. There were no statistically significant differences between the two groups at baseline as reported in Table 1.
Table 1.
Baseline relevant clinical characteristics, body composition, and gonadal hormone levels between AI and no-AI users (no statistically significant differences)
| Parameters | No AIs mean ± SE (median) (n = 71) | AIs mean ± SE (median) (n = 11) |
|---|---|---|
| Age (yr) | 49.78 ± 0.61 | 51.18 ± 2.14 |
| Years postmenopausal | 3.15 ± 0.22 | 3.39 ± 0.61 |
| ERAA | 50 | NA |
| No adjuvant hormonal therapy | 21 | NA |
| Body composition | ||
| Total body mass (kg) | 74.9 ± 2.0 | 76.4 ± 4.1 |
| Lean body mass (kg) | 44 ± 0.8 | 45.6 ± 1.9 |
| Total body fat (%) | 37.70 ± 0.78 | 36.99 ± 1.21 |
| Gonadal hormones | ||
| Testosterone total (ng/ml) | 0.28 ± 0.02 | 0.30 ± 0.03 |
| Testosterone free (pg/ml) | 1.27 ± 0.07 | 1.49 ± 0.16 |
| SHBG (nmol/l) | 157.27 ± 10.46 | 97.35 ± 15.21 |
| Estradiol total (pg/ml) | 27.68 ± 5.68 | 17.34 ± 8.55 |
For additional baseline characteristics, please refer to a prior publication [5]
SE Standard error, SHBG sex hormone binding globulin, AI aromatase inhibitor
Changes in body composition (Fig. 1)
Fig. 1.
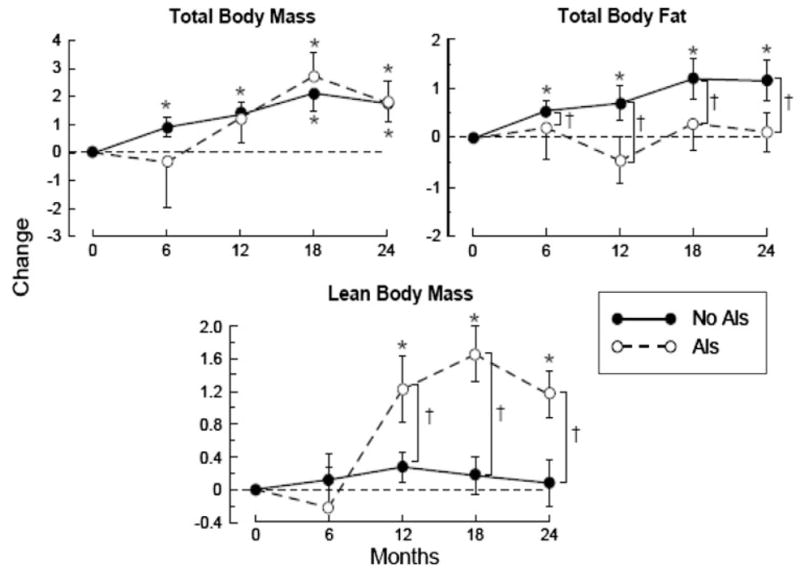
Changes (mean and standard error) in total body mass (kg), total body fat (%), and lean body mass (kg) over 24 months (* P ≤ 0.05 change from baseline, †P ≤ 0.05 between-group comparison). AI aromatase inhibitor
The total body mass increased 1.79 ± 0.74 kg (P < 0.05) from baseline over 24 months for women who were on AIs, as well as those who were not (1.76 ± 0.66 kg), without any significant difference between these groups (Fig. 1). Total body fat increased 1.2 ± 0.4% (P < 0.05) for women not on AIs while it remained stable in AI users resulting in a significant difference (P < 0.05) between the AI and no-AI users over 24 months. The opposite was observed for the lean body mass changes over 24 months: AI users displayed a 1.16 ± 0.28 kg increase in lean body mass compared to both baseline and no-AI users (P < 0.05).
Changes in gonadal hormone levels
There were no significant changes between the AI users and no-AI users for total testosterone and estradiol values at 24 months (Fig. 2). However, the free testosterone levels were significantly higher and the SHBG levels were significantly lower compared to no-AI users at 24 months (P < 0.01).
Fig. 2.
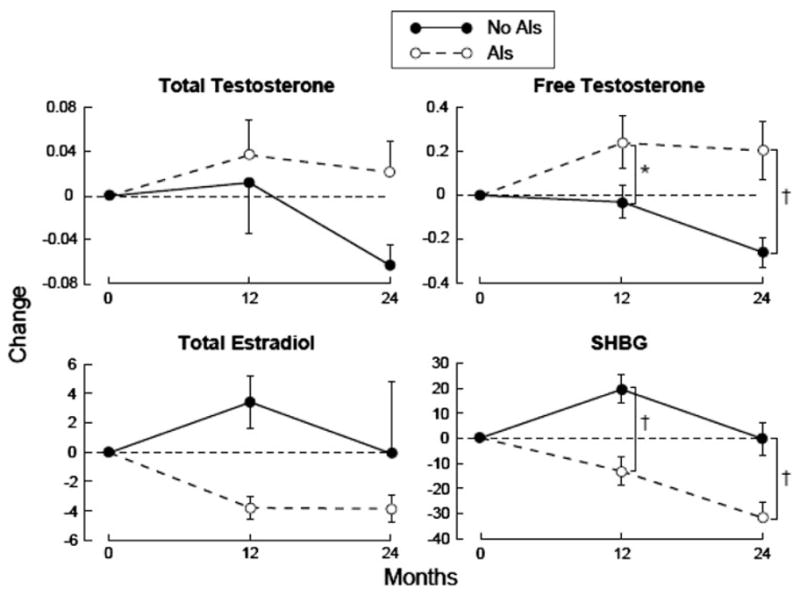
Changes (mean and standard error) in total testosterone (ng/ml), free testosterone (pg/ml), total estradiol (pg/ml), and SHBG (nmol/l) over 24 months (between-group comparisons: *P ≤ 0.05, †P ≤ 0.01). AI Aromatase inhibitor, SHBG sex hormone binding globulin
Interpretation
This secondary analysis of the REBBeCA study demonstrated that chemotherapy-induced, newly postmenopausal women with breast cancer increased their total body mass significantly over 24 months. Those who used AIs demonstrated stable total body fat mass, increased lean body mass as well as free testosterone levels, and decreased SHBG levels, compared to no-AI users.
The effect of ERAAs or AIs on body composition has been relatively underexplored. Raloxifene, an ERAA, has revealed mixed results on body composition. Two randomized clinical trials demonstrated that it exerts estrogenic agonistic effects on body composition as early as after 8 weeks of drug therapy (i.e., shifts body composition from android to gynoid distribution) [7, 8]. However, a third randomized trial did not detect an effect of raloxifene on body composition over 12 months, but the baseline values of body composition differed between controls and women on raloxifene [9]. A large, 24 month, randomized clinical trial is underway [10]. The effect of tamoxifen, another ERAA, on body composition has also been inconsistent. A cross-sectional study revealed that post-menopausal tamoxifen users, compared to postmenopausal controls, had higher values for CT-scan derived liver density and visceral fat areas [11]. However, a randomized clinical trial did not demonstrate an effect of tamoxifen on body weight or body fat distribution [12]. The effect of an aromatase inhibitor on body composition was examined in a trial, which randomized postmenopausal women, who had received at least 2 years of treatment with tamoxifen, to either continuation treatment with tamoxifen versus a switch to an AI (exemestane) [13]. This trial revealed that the women who were randomized to continuation treatment with tamoxifen did not demonstrate a change in fat mass and ratio of “fat free mass over fat mass” over 1 year. However, the women who were randomized to exemestane revealed a decrease in fat mass and an increase in ratio of “fat free mass/fat mass”. The author did not provide an explanation for the finding that AIs do not decrease muscle mass, as was expected based on the relationship between estrogens and muscle mass [14], but increase muscle mass. Although we did find a statistically significant alteration in body composition, the clinical relevance of an increase of 1.16 kg in lean mass for women on AIs, or an increase of 1.16% of body fat for women not on AIs, is not known.
To the best of our knowledge, there are no additional publications available reporting the effect of AIs on body composition. We hypothesized that the increase in lean body mass in women on AIs is related to a relative increase in male gonadal hormones. AIs inhibit aromatase, an enzyme which is responsible for the majority of estrogen production in postmenopausal women by converting male gonadal hormones peripherally [2]. As a result, inhibition of aromatase leads to lower estrogen levels, but can also theoretically contribute to accumulation of male gonadal hormones, as we have shown (Fig. 2). The effect of AIs on male gonadal hormones in postmenopausal women was assessed by Dowsett et al. Both these investigations reported no changes of male gonadal hormone levels in women on anastrazole [15] (but duration of treatment was only up to 14 days) and letrozole [16] (duration of treatment was 12 months). A positive interaction between androgens and muscle mass has been established in men and is only beginning to be explored in women [17, 18].
The finding of an increase in testosterone may have clinical relevance. If a relative increase of testosterone can induce a 1.16 kg increase in lean body mass, it warrants our attention to also explore its effects on cardiovascular risk factors and disease. The pro-atherogenic role of androgens, relative to estrogens, in female cardiovascular disease is being increasingly recognized [19–21]. Similarly, we are only starting to understand that SHBG per se can play an active role as well: decreased levels of SHBG increase risk of diabetes mellitus II and vice versa [22, 23]. Therefore, the observation that women on AIs have a triple insult is potentially worrisome (higher levels of androgens, lower levels of SHBG and estrogens). Currently, the effects of AIs on lipid profile and cardiovascular risk remain inconclusive [24–27] and require further exploration especially in light of a recent publication which reported higher mortality in non-relapsed breast cancer patients on AIs [28].
There are several limitations to this study. A relatively small proportion of women in this study were on AIs. Since this represents a secondary analysis we were unable to control for many factors such as lifestyle habits and the exact timing of initiation/modification/discontinuation of hormonal therapy. Body composition had been assessed via DXA methodology which is an acceptable method but we could not assess compartmental information, which may possibly underlie our observation that total body fat did not change significantly over 24 months in women on AIs [29]. Similarly, DXA methodology does not allow us to differentiate between muscle and water within lean body mass. This knowledge may be essential, since hormonal therapy has been shown to be able to affect water content [30]. Therefore, the effect of AIs on lean body mass cannot be assumed to be related to a change in muscle mass only, without also taking into account water content. Gonadal hormone levels were measured using RIA methodology. Time-fluctuation may have interfered with these assessments. In addition, lower levels of estrogen are near the detection limit of RIA methodology, which may explain why we observed a trend toward lower estrogens in AI-users, which did not reach statistical significance. In addition, systemic gonadal hormone levels alone may not provide sufficient information, since the microenvironment needs to be taken into account as well [31]. Finally, similar to Francini et al. [13], our data do not provide an insight into the “chicken and egg” phenomenon, i.e., whether changes in body composition precede gonadal hormone level changes, or vice versa, or a combinational vicious cycle.
The strengths of this study include that this is the first study revealing the effect of AIs on body composition (and gonadal hormone profile). The study was performed at a single center resulting in the usage of one DXA machine and a limited number of technologists, allowing us to obtain a better estimate of true treatment effect. The duration of follow-up was 2 years, which provides a good insight into the long-term effects of AIs on body composition.
We conclude that chemotherapy-induced postmenopausal breast cancer patients over 24 months demonstrate an increase of total body mass, independent of AI usage. The use of AIs is associated with a maintenance of total body fat, an increase in lean body mass and free testosterone, and a decrease in SHBG levels compared to no-AI users at 24 months. These observations provide an important insight into non-cancer effects of AIs, which may have long-term consequences. The mechanisms and implications of these changes require further exploration.
Acknowledgments
All funding sources supporting publication of a work or study are as follows. NIH/NIDDKD (K24: DK062895-05): awarded to Dr. Greenspan. A Procter and Gamble and Sanofi-Aventis noncompany-sponsored trial grant: awarded to Dr. Greenspan. NIH/NCRR (M01-RR00056): awarded to the University of Pittsburgh. NIH/NCRR (RR024154): awarded to Dr. Steven E. Reis. John A. Hartford foundation (2004-0485): provided support for assays. None of these funding agencies were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript. Risedronate, matching placebo, calcium, and vitamin D supplements were provided by Procter and Gamble, Inc. We are indebted to the nursing, professional, laboratory, dietary, administrative, and study staff of the Clinical Translational Research Center of Montefiore University Hospital and Osteoporosis Prevention and Treatment Center at the University of Pittsburgh. We acknowledge the members of the Data and Safety Monitoring Board for their oversight of the study.
Footnotes
Conflict of interest Dr Greenspan has received grant-support from Procter and Gamble, Inc., Sanofi-Aventis, Amgen, and Lilly. Dr Greenspan also serves as a consultant for Merck. Dr. Perera has received funding in the past from Eli Lilly and Co., Ortho Biotech, LLC, Teva Neuroscience for observational research. All other authors have no conflict of interest.
Contributor Information
G. J. van Londen, Email: vanLondenJ@upmc.edu, Medicine, University of Pittsburgh, 3471 Fifth Ave, Suite 500 Kaufmann Medical Bldg, Pittsburgh, PA 15213, USA
S. Perera, Medicine, University of Pittsburgh, 3471 Fifth Ave, Suite 500 Kaufmann Medical Bldg, Pittsburgh, PA 15213, USA
K. Vujevich, Medicine, University of Pittsburgh, 3471 Fifth Ave, Suite 500 Kaufmann Medical Bldg, Pittsburgh, PA 15213, USA
P. Rastogi, Medicine, University of Pittsburgh, 3471 Fifth Ave, Suite 500 Kaufmann Medical Bldg, Pittsburgh, PA 15213, USA
B. Lembersky, Medicine, University of Pittsburgh, 3471 Fifth Ave, Suite 500 Kaufmann Medical Bldg, Pittsburgh, PA 15213, USA
A. Brufsky, Medicine, University of Pittsburgh, 3471 Fifth Ave, Suite 500 Kaufmann Medical Bldg, Pittsburgh, PA 15213, USA
V. Vogel, Cancer Institute, Geisinger Medical Center, Danville, PA, USA
S. L. Greenspan, Medicine, University of Pittsburgh, 3471 Fifth Ave, Suite 500 Kaufmann Medical Bldg, Pittsburgh, PA 15213, USA
References
- 1.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348(24):2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 3.Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28(3):509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 4.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 5.Greenspan SL, Brufsky A, Lembersky BC, Bhattacharya R, Vujevich KT, Perera S, Sereika SM, Vogel VG. Risedronate prevents bone loss in breast cancer survivors: a 2-year, randomized, double-blind, placebo-controlled clinical trial. J Clin Oncol. 2008;26(16):2644–2652. doi: 10.1200/JCO.2007.15.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuerst T, Genant HK. Evaluation of body compositlon and total bone mass with the hologic QDR 4500. Osteo Int. 1996;6(Suppl 1):203. [Google Scholar]
- 7.Lee CC, Kasa-Vubu JZ, Supiano MA. Differential effects of raloxifene and estrogen on insulin sensitivity in postmenopausal women. J Am Geriatr Soc. 2003;51(5):683–688. doi: 10.1034/j.1600-0579.2003.00214.x. [DOI] [PubMed] [Google Scholar]
- 8.Francucci CM, Daniele P, Iori N, Camilletti A, Massi F, Boscaro M. Effects of raloxifene on body fat distribution and lipid profile in healthy post-menopausal women. J Endocrinol Invest. 2005;28(7):623–631. doi: 10.1007/BF03347261. [DOI] [PubMed] [Google Scholar]
- 9.Tommaselli GA, Di Carlo C, Di Spiezio Sardo A, Bifulco G, Cirillo D, Guida M, Capasso R, Nappi C. Serum leptin levels and body composition in postmenopausal women treated with tibolone and raloxifene. Menopause. 2006;13(4):660–668. doi: 10.1097/01.gme.0000227335.27996.d8. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen DE, Samson MM, Schouw YT, Grobbee DE, Verhaar HJ. Efficacy of tibolone and raloxifene for the maintenance of skeletal muscle strength, bone mineral density, balance, body composition, cognitive function, mood/depression, anxiety and quality of life/well-being in late postmenopausal women >/=70 years: study design of a randomized, double-blind, double-dummy, placebo-controlled, single-center trial. Trials. 2008;9:32. doi: 10.1186/1745-6215-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen MC, Stewart RB, Banerji MA, Gordon DH, Kral JG. Relationships between tamoxifen use, liver fat and body fat distribution in women with breast cancer. Int J Obes Relat Metab Disord. 2001;25(2):296–298. doi: 10.1038/sj.ijo.0801488. [DOI] [PubMed] [Google Scholar]
- 12.Grey A, Stapleton J, Evans M, Reid I. The effect of the anti-estrogen tamoxifen on cardiovascular risk factors in normal postmenopausal women. J Clin Endocrinol Metab. 1995;80(11):3191–3195. doi: 10.1210/jcem.80.11.7593425. [DOI] [PubMed] [Google Scholar]
- 13.Francini G, Petrioli R, Montagnani A, Cadirni A, Campagna S, Francini E, Gonnelli S. Exemestane after tamoxifen as adjuvant hormonal therapy in postmenopausal women with breast cancer: effects on body composition and lipids. Br J Cancer. 2006;95(2):153–158. doi: 10.1038/sj.bjc.6603258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010;40(1):41–58. doi: 10.2165/11319760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Yates RA, Dowsett M, Fisher GV, Selen A, Wyld PJ. Arimidex (ZD1033): a selective, potent inhibitor of aromatase in postmenopausal female volunteers. Br J Cancer. 1996;73(4):543–548. doi: 10.1038/bjc.1996.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajetta E, Zilembo N, Dowsett M, Guillevin L, Di Leo A, Celio L, Martinetti A, Marchiano A, Pozzi P, Stani S, et al. Double-blind, randomised, multicentre endocrine trial comparing two letrozole doses, in postmenopausal breast cancer patients. Eur J Cancer. 1999;35(2):208–213. doi: 10.1016/s0959-8049(98)00392-x. [DOI] [PubMed] [Google Scholar]
- 17.Bhasin S, Woodhouse L, Storer TW. Proof of the effect of testosterone on skeletal muscle. J Endocrinol. 2001;170(1):27–38. doi: 10.1677/joe.0.1700027. [DOI] [PubMed] [Google Scholar]
- 18.Miller KK. Androgen deficiency: effects on body composition. Pituitary. 2009;12(2):116–124. doi: 10.1007/s11102-008-0121-7. [DOI] [PubMed] [Google Scholar]
- 19.Ling S, Komesaroff PA, Sudhir K. Cardiovascular physiology of androgens and androgen testosterone therapy in post-menopausal women. Endocr Metab Immune Disord Drug Targets. 2009;9(1):29–37. doi: 10.2174/187153009787582414. [DOI] [PubMed] [Google Scholar]
- 20.Torrens JI, Sutton-Tyrrell K, Zhao X, Matthews K, Brockwell S, Sowers M, Santoro N. Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: study of Women’s Health Across the Nation. Menopause. 2009;16(2):257–264. doi: 10.1097/gme.0b013e318185e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168(14):1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361(12):1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry JR, Weedon MN, Langenberg C, Jackson AU, Lyssenko V, Sparso T, Thorleifsson G, Grallert H, Ferrucci L, Maggio M, et al. Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum Mol Genet. 2010;19(3):535–544. doi: 10.1093/hmg/ddp522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdulhaq H, Geyer C. Safety of adjuvant endocrine therapy in postmenopausal women with breast cancer. Am J Clin Oncol. 2008;31(6):595–605. doi: 10.1097/COC.0b013e31816d9171. [DOI] [PubMed] [Google Scholar]
- 25.Ewer MS, Gluck S. A woman’s heart: the impact of adjuvant endocrine therapy on cardiovascular health. Cancer. 2009;115(9):1813–1826. doi: 10.1002/cncr.24219. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi S, Verma S. Aromatase inhibitors and cardiac toxicity: getting to the heart of the matter. Breast Cancer Res Treat. 2007;106(1):1–9. doi: 10.1007/s10549-006-9470-y. [DOI] [PubMed] [Google Scholar]
- 27.Lewis S. Do endocrine treatments for breast cancer have a negative impact on lipid profiles and cardiovascular risk in postmenopausal women? Am Heart J. 2007;153(2):182–188. doi: 10.1016/j.ahj.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 28.Seruga B, Tannock IF. Up-front use of aromatase inhibitors as adjuvant therapy for breast cancer: the emperor has no clothes. J Clin Oncol. 2009;27(6):840–842. doi: 10.1200/JCO.2008.19.5594. [DOI] [PubMed] [Google Scholar]
- 29.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity. 2009;18(3):604–610. doi: 10.1038/oby.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobsen D, Samson M, Emmelot M, Verhaar H. Raloxifene and body composition and muscle strength in post-menopausal women: a randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2009;162:371–376. doi: 10.1530/EJE-09-0619. [DOI] [PubMed] [Google Scholar]
- 31.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2010;30(4):343–375. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]


