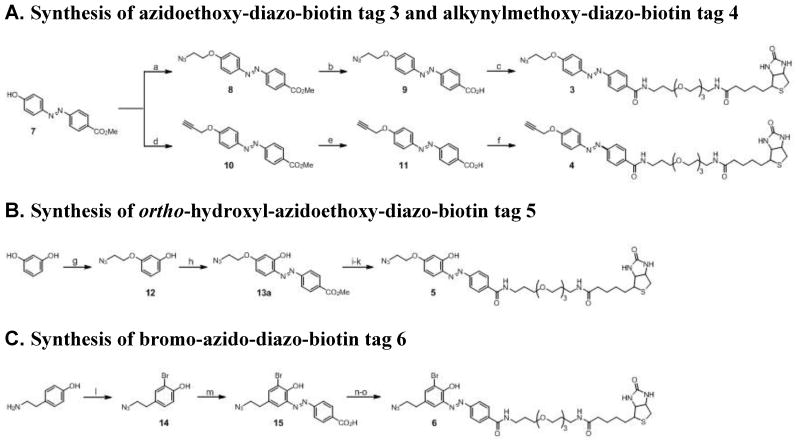
Figure 2. Synthesis of the second-generation diazobenzene-based cleavable affinity tags (3–6).
(a) 2-azidoethyl tosylate, K2CO3, DMF, 0 °C then rt, 85 %; (b) LiOH, THF/H2O (v/v = 1/1), pH = 12.0, 90%; (c) i. oxalyl chloride, cat. DMF, CH2Cl2; ii. biotin-PEG-NH2, Et3N, CH2Cl2, 55 %; (d) propargyl bromide, K2CO3, DMF, rt, 70 %; (e) LiOH, THF/H2O (v/v = 1/1), pH = 12, 90%; (f) i. oxalyl chloride, cat. DMF, CH2Cl2; ii. biotin-PEG-NH2, Et3N, CH2Cl2, 39 %; (g) resorcinol, 2-azidoethyl tosylate, EtOH, KOH(aq), reflux, 60%; (h) i. methyl 4-aminobenzoate, 6 N HCl(aq), NaNO2, 0 °C, 15 min; ii. K2CO3, THF, pH 8.0, 0 °C then rt, 5%; (i) LiOH, THF/H2O (v/v = 1/1), pH = 12.0, >95%; (j) N-hydroxysuccinimide, DCC, THF, 2.5 h; (k) biotin-PEG-NH2, DMF, 4 h, 65% over 2 steps; (l) Br2, AcOH, 84%; (m) i. 4-aminobenzoic acid, 6 N HCl(aq), NaNO2, 0 °C, 25 min; ii. K2CO3, THF, pH 8.0, 0 °C then rt, 8–15%; (n) N-hydroxysuccinimide, DCC, THF, 3 h; (o) biotin-PEG-NH2, DMF, 4 h, 53% over 2 steps. See also Figure S4.