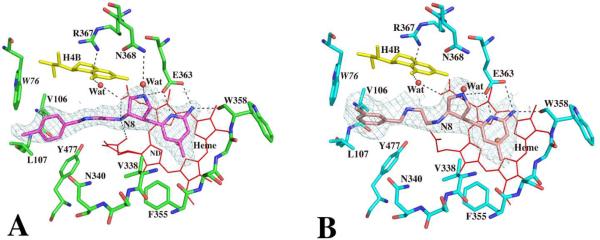
Fig. 4.
Active site structures of the wild type eNOS with inhibitor 6 (panel A) or 7 (panel B) bound. Also shown around the inhibitor is the Fo – Fc omit map contoured at 3.0σ. Residue Trp76 belongs to the neighboring subunit. Alternate conformations of heme propionate off the pyrrole ring D are also depicted.