Abstract
Soil microbial communities mediate the decomposition of soil organic matter (SOM). The amount of carbon (C) that is respired leaves the soil as CO2 (soil respiration) and causes one of the greatest fluxes in the global carbon cycle. How soil microbial communities will respond to global warming, however, is not well understood. To elucidate the effect of warming on the microbial community we analyzed soil from the soil warming experiment Achenkirch, Austria. Soil of a mature spruce forest was warmed by 4 °C during snow-free seasons since 2004. Repeated soil sampling from control and warmed plots took place from 2008 until 2010. We monitored microbial biomass C and nitrogen (N). Microbial community composition was assessed by phospholipid fatty acid analysis (PLFA) and by quantitative real time polymerase chain reaction (qPCR) of ribosomal RNA genes. Microbial metabolic activity was estimated by soil respiration to biomass ratios and RNA to DNA ratios. Soil warming did not affect microbial biomass, nor did warming affect the abundances of most microbial groups. Warming significantly enhanced microbial metabolic activity in terms of soil respiration per amount of microbial biomass C. Microbial stress biomarkers were elevated in warmed plots. In summary, the 4 °C increase in soil temperature during the snow-free season had no influence on microbial community composition and biomass but strongly increased microbial metabolic activity and hence reduced carbon use efficiency.
Keywords: Soil warming, Microbial biomass, Microbial community structure, PLFA, rRNA genes
Highlights
► No warming effects on microbial biomass C and N. ► No warming effects on major microbial communities. ► Increase of stress biomarker in warmed soil. ► Warming caused a strong increase in microbial metabolic activity (soil respiration per biomass).
1. Introduction
Global warming is considered to promote the decomposition of SOM, and thereby to increase the C flux from soil to the atmosphere (Cox et al., 2000; IPCC, 2007; Trumbore et al., 1996). SOM is decomposed by heterotrophic microorganisms, which due to the enormous C fluxes they generate, are one of the main drivers of the global C cycle. How these microorganisms respond to warming, however, is difficult to predict as a set of environmental and biological factors interact during SOM decomposition. The overall warming response of SOM decomposition will be determined by the temperature sensitivity of the decomposers, substrate availability, interactions with aboveground processes and other environmental drivers such as soil moisture, and also by potential adaptations of microbial physiology (Allison et al., 2010; Davidson and Janssens, 2006; De Deyn et al., 2008; Knorr et al., 2005). So far, most effort has been made to understand how increased soil temperature influences the CO2 efflux from soil. In comparison, physiology and composition of the microbial decomposer community were rarely studied. There is evidence that warming potentially alters decomposer physiology and accordingly the CO2 efflux from soil (Allison et al., 2010; Balser and Wixon, 2009; Bárcenas-Moreno et al., 2009; Bradford et al., 2008; Zogg et al., 1997). The mechanisms behind temperature adaptations of soil microbes could be physiological adaptations of single species (Malcolm et al., 2008) or species shifts within the microbial community. As various decomposing microbes differ within their ability/strategy to efficiently utilize SOM (Balser and Wixon, 2009; Keiblinger et al., 2010; Lipson et al., 2009; Liptzin et al., 2009; Monson et al., 2006), shifts within the community structure may affect decomposition rates and CO2 production. Lipson et al. (2009) and Keiblinger et al. (2010), for example, showed that fungi form more biomass from the same amount of added substrate than bacteria, thus they use organic substrates more efficiently. Bacteria on the other hand showed higher growth rates and lower yields suggesting that they were important for determining heterotrophic soil respiration rates. Fungi were further found to dominate during early stages of plant residue decomposition (Berg et al., 1998; McMahon et al., 2005). Gram-positive and Gram-negative bacteria showed different patterns in substrate preference. Gram-positive bacteria were found to be dominant in soils with low substrate availability and in deeper soil layers (Fierer et al., 2003), while Gram-negative bacteria were found to dominate soils with high availability of easily decomposable substrate (Kramer and Gleixner, 2006). Archaea were found abundant in many soils and important in CH4 and N dynamics (Leininger et al., 2006). There is evidence that Archaea are able to perform heterotrophic or mixotrophic catabolism (Jia and Conrad, 2009) but their role in SOM decomposition is unclear.
Although functional traits (organisms with same physiological pathway) are not strictly related to the taxonomic units mentioned above, the relative abundance of fungi, Gram-positive or Gram-negative bacteria, and other microbial groups can give some insights in the physiological capacity of the soil microbial community. A good example was given by Lipson et al. (2009) who showed that distinct microbial winter- and summer-communities differed in growth kinetics, biomass-specific respiration rates and temperature sensitivity of soil respiration. A temperature difference between winter and summer however is not comparable with the expected temperature increase due to global warming. In order to simulate anticipated global warming (1–5 °C) a large array of field and lab warming studies have been performed. Although microbial assessments were not regularly undertaken, some general patterns were observed. A common finding of most warming studies was that warming did not increase microbial biomass in soil (Biasi et al., 2008; Feng and Simpson, 2009; Rinnan et al., 2007, 2008, 2009; Vanhala et al., 2011; Zhang et al., 2005; Zogg et al., 1997). Depending on the duration of the warming treatment, microbial biomass either remained at steady levels or decreased. Regarding microbial community composition, the picture was more complex. Dependent on the ecosystem observed, the duration of warming and the experimental warming approach, changes in microbial community composition were observed in terms of increased fungal abundance, decreased fungal abundance, increased abundance of Gram-positive bacteria, decreased abundance of Gram-positive bacteria, decreased abundance of Gram-negative bacteria, or not at all (Biasi et al., 2005; Castro et al., 2010; Feng and Simpson, 2009; Frey et al., 2008; Karhu et al., 2010; Rinnan et al., 2007, 2008, 2009; Vanhala et al., 2011; Zogg et al., 1997).
In the present study, we took the opportunity to sample soil from the forest soil warming experiment in Achenkirch, Austria, (Schindlbacher et al., 2009). Soil was warmed 4 °C above ambient throughout growing seasons since 2004. CO2 flux rates were regularly measured. To assess microbial biomass and community composition, repeated soil sampling from control and warmed plots took place in the fourth and fifth year of artificial warming. According to enzyme kinetics (Davidson and Janssens, 2006), we hypothesized that (I) soil warming strongly enhanced microbial metabolic activities. We further hypothesized that (II) increased soil temperatures generated advantages for specific microbial groups better adapted to warmer conditions and hence caused shifts in the microbial community composition. A decrease in fungal abundance, as observed in related studies (Frey et al., 2008; Vanhala et al., 2011) was anticipated.
2. Materials & methods
2.1. Site description
The study site was located at 910 m a.s.l. in the North Tyrolean Limestone Alps, near Achenkirch, Austria (11°38′21″ East; 47°34′50″ North). The 130 year-old mountain forest consists of Norway spruce (Picea abies) with inter-spread of European beech (Fagus sylvatica) and silver fir (Abies alba). Climate at the site was cool, humid, with maximum precipitation during summer. Snow-free periods were typically from April or May to November or December. Mean annual air temperature and precipitation were 5.7 °C and 1480 mm, respectively (1987–2007, ZAMG). Soils were shallow Chromic Cambisols and Rendzic Leptosols with high spatial variability. The dominant humus form was mull followed by thick A-horizons that reach up to 10–20 cm in Chromic Cambisols and 60 cm in Rendzic Leptosols. Dolomite formed the bedrock. The soil pH was slightly above 6 and the C/N ratio was 15–18. A detailed site description is given in Herman et al. (2002).
2.2. Soil warming and soil respiration measurements
Three experimental plots with 2 × 2 m warmed- and control-subplots were installed in 2004. Within the heated plots resistance heating cables (Etherma, Salzburg) were installed at 3 cm soil depth and with 7.5 cm space between the cable lines. To account for potential disturbance effects, the same cables were installed in control plots but not heated. The temperature difference between control and warmed plots was set to 4 °C at 5 cm soil depth. Soil was warmed during snow-free seasons. Soil temperature and moisture of each subplot were measured year-round at 5 and 15 cm soil depth and data were stored on data-loggers at half-hourly intervals. Soil respiration was measured manually every second week during the growing seasons and every third week during snow-cover. A detailed description of the experimental setup and soil respiration measurements is given in Schindlbacher et al. (2009) and Schindlbacher et al. (2007).
2.3. Soil sampling
For soil analysis the Ah horizon (0–5 cm) was sampled with a 3 cm diameter soil-corer. At warmed plots, soil samples were taken exactly half way between two heating-cable lines to assure that all samples received the same heat input. In order to represent different seasonal conditions the samples were taken on the following dates in 2008: 14 February, 6 May, 29 June, 23 September, 18 November, and in 2009: 18 March, 17 June, 10 August and 22 September. The last samples were taken on 23 March 2010. Three replicates from each of the six 2 × 2 m subplots (three warmed and three controls) were analyzed for the sample dates 6 May, 29 June, 18 November 2008 and 18 March 2009. Because within-subplot variability was low, we pooled the three randomly taken samples across each subplot to one mixed sample for analysis during subsequent sampling dates. The samples were stored in cooling boxes and transported to the laboratory in Vienna where the soil samples were homogenized with 5 mm mesh sized sieves and frozen at −20 °C. Prior to laboratory analysis, soil water content was determined gravimetrically by drying soil samples at 105 °C for 24 h (Schinner et al., 1996). For qPCR analysis two samples of about 100 g fresh soil were collected from each subplot and homogenized. Aliquots were immediately frozen in liquid nitrogen and stored at −80 °C.
2.4. Microbial analysis
Microbial analysis focused on the total microbial biomass and the abundance of specific microbial groups. To assess microbial biomass C and N, we applied chloroform-fumigation-extraction. Specific microbial groups were separated by PLFA analysis and alternatively by qPCR. Microbial activity was estimated according to respiration to biomass ratios and RNA to DNA ratios.
2.4.1. Chloroform fumigation extraction (CFE)
Microbial biomass C and N were determined using a modified version of the CFE method (Schinner et al., 1996). Eleven grams of homogenized soil were weighed into 100 ml Erlenmeyer flasks to be chloroform fumigated and 5 g were weighed into plastic beakers as control samples. The soil samples in the Erlenmeyer flasks were kept inside a desiccator with sodium lime and wet filter papers within a chloroform atmosphere for 24 h at 25 °C. After fumigation the samples were split into two 5 g samples and the remaining 1 g of soil was withdrawn. Twenty five ml of 2 M KCl solution were added to the samples that were then shaken for 30 min and afterward filtered through N-free filters. Control samples were processed using the same procedure. The C and N content of the KCl extracts were measured with a TOC-V CPH E200 V soluble analyzer linked with a TN-unit TNM-1220 V (Shimadzu, Kyoto, Japan). Samples were measured on four different days. For calibration a dilution series of a standard stock solution was added. Microbial biomass C and N contents in μg g−1 dry matter were calculated by subtracting C and N contents of the control sample from mean C and N contents of the two fumigated samples.
2.4.2. Phospholipid fatty acid analysis (PLFA)
In order to investigate the soil microbial community, soil samples were characterized by PLFA analysis using a modified method after Frostegård et al. (1991) and Hackl et al. (2005). Water contents of the soil samples were measured prior to the procedure for adjusting the method and for further calculations. 1.5 g of fresh soil sample were then extracted with a chloroform:methanol:citrate buffer mixture (1:2:0.8, v/v/v). The lipids were separated into neutral lipids, glycolipids and phospholipids on a silica acid column. Phospholipids were subjected to a mild alkaline methanolysis. The extraction with fatty acid methyl esters was analyzed with a HP 6890 Series gas chromatograph instrument equipped with a 7683 Series injector and auto sampler on a HP - 5 capillary column (50.0 m, 0.20 mm, 0.33 μm) and detected with a FID (flame ionization detector) using Helium as carrier gas (Hewlett Packard, Wilmington, Delaware, USA). Temperatures of the injector and the detector were 280 °C and 350 °C, respectively. The injected sample volume was 1 μl (spitless mode injection). The initial oven temperature of 70 °C was maintained for 1.5 min, and then subsequently raised by 30 °C min−1 to 160 °C, by 4 °C min−1 to 270 °C, and by 30 °C min−1 to the final temperature of 300 °C, which was held for 39 min. All GC measurements included a blank sample with the internal standard (peak 19:0, nonadeconoate fatty acid) - one sample with a standard qualitative bacterial acid methyl esters mix (BAC mix) and one sample with a standard qualitative fatty acid methyl esters mix (FAME mix; both Sigma Aldrich Co., St. Louis, MO) for easier identification of the fatty acid peaks. All chemicals used were of high purity and suitable for GC measurements. In total 32 peaks were detected per sample. The areas measured by GC-FID were used for calculating the abundance of PLFA markers in nmol g−1 dry weight of soil sample and those values were used for further analysis. For characterizing the community structure we used the terminal-branched saturated PLFA peaks i15:0, a15:0, i16:0, a16:0, i17:0, a17:0 as marker for Gram-positive bacteria (Zelles, 1997). The mono-unsaturated and cyclopropyl saturated peaks 16:1ω5, 16:1ω9, 17:1ω9, cy17:0, 18:1ω11, cy19:0 were used as indicators for Gram-negative bacteria and the PLFA peaks 14:0, 15:0, 17:0 for unspecific bacteria (Fierer et al., 2003; Frostegård et al., 1993; Zelles, 1997; Zogg et al., 1997). Furthermore, 18:2ω6,9 was used as fungal PLFA marker (Frostegård and Bååth, 1996; Högberg, 2006; Olsson, 1999; Zogg et al., 1997). The methylic, mid-chain-branched saturated PLFA peaks 10Me16:0, 10Me17:0, 10Me18:0 were used as indicators for actinomycetes (Frostegård et al., 1993). As for arbuscular mycorrhiza peak we used 16:1ω11 (Olsson, 1999) and the peaks 20:4ω6 and 20:0 were used as indicators for protozoa (White et al., 1996). Total bacterial PLFAs were calculated as the sum of Gram-negative, Gram-positive and unspecific bacteria. The bacteria to fungi ratio of PLFAs was calculated by dividing the sum of all bacterial PLFA marker through the fungal PLFA marker (Bååth and Anderson, 2003) and was used as the relative abundance of these two microbial groups. Furthermore we used an indicator for stress by dividing the peak for the fatty acid cy17:0 through its metabolic precursor fatty acid 16:1ω7 for each sample (Feng and Simpson, 2009; Kaur et al., 2005).
2.4.3. Quantitative real time PCR (qPCR)
We analyzed samples from sampling dates: May 2008 and July 2008. DNA and RNA were co-extracted from 0.5 g frozen soil as described by Griffiths et al. (2000) and purified with custom Sepharose® (Sigma) CL6B columns. Each extract was partitioned into a DNA subsample, and an RNA subsample. RNA subsamples were treated with DnaseI (MICROBExpress TM, Ambion) and reverse transcribed using the Iscript select cDNA synthesis kit (Bio-rad). qPCR was carried out in an iCycler iQ Multicolor Real Time PCR Detection System (BIO-Rad Laboratories) in 25 μl reaction mixtures containing 1× IQ TM SYBR-Green Supermix (BIO-Rad 179 Laboratories), 25 μg BSA and of 2 μM specific primers. Sixty ng of total nucleic acids were used as template for quantification of actinobacterial and archaeal 16S rDNA and fungal 28S rDNA. For quantification of total bacterial 16S rDNA, which was present in much higher concentration, template input was reduced to 15 ng per reaction. A cDNA equivalent of 7 ng total nucleic acids was used as template for the quantification of bacterial 16S rRNA. Primers Eub338 (Lane, 1991) and Eub518r (Muyzer et al., 1993) were used for the analysis of bacteria, while Actino235 (Stach et al., 2003) and Eub518r for actinobacteria, Ar109f and Ar912rt (Lueders and Friedrich, 2000) for Archaea, CTB6 and TW13 (http://pmb.berkeley.edu/∼bruns/tour/primers.html#28s) for fungi. Two replicate qPCR reactions per soil sample were analyzed with each primer set. The cycling conditions were 3 min at 95 °C followed by 40 cycles of 30 s at 95 °C, 35 s at the specific annealing temperature and 45 s at 72 °C. Data were collected at 72 °C. Annealing temperatures were optimized for each reaction to obtain highly specific PCR products. The specific annealing temperatures were 54 °C for bacteria and actinobacteria, 52 °C for Archaea and 58 °C for fungi. Standards for qPCR were generated from Escherichia coli clones containing plasmids that carried the respective PCR target fragment as insert. Plasmids were isolated using Easy Prep Pro (Biozym), quantified in a NanoDrop photometer (NanoDrop Technologies, Wilmington) and serial dilutions containing known numbers of plasmids were prepared. The specificity of the PCR product was confirmed by melting curve analysis. qPCR products were also analyzed on agarose gel to confirm the exclusive amplification of the desired fragment. Optimal template dilutions were determined by checking PCR inhibition in serial dilutions from three different samples. Amplification efficiencies, quantification equations and detection limits of qPCR reactions are given in the supplementary material.
Absolute cell numbers in the soil cannot be directly deduced from ribosomal gene copies detected in the applied qPCR approach. DNA of certain taxa may have been preferentially isolated and amplified. The PCR primers may not have perfectly matched all members of the respective target group present in the soil, which may have selectively reduced amplification efficiency of certain targets (Bru et al., 2008; Sipos et al., 2007). More over the number of ribosomal gene copies varies among microorganisms between 1 and 15 copies per genome. We therefore used qPCR only to detect potential differences between individual microbial groups in soil samples from warmed and control plots rather than to compare the absolute abundance of different taxonomic groups in the soil.
2.4.4. Microbial activity
We determined respiration to biomass ratios as measures of microbial metabolic activity. For that, we divided field soil respiration rates (μg CO2-C m−2 h−1) by corresponding microbial biomass C concentrations (μg C g−1 dw). Respiration to biomass ratios calculated this way are not metabolic quotients (the amount of CO2-C produced per unit microbial biomass C (Anderson and Domsch, 1978)) because field soil respiration consists of a heterotrophic and an autotrophic component. At our site, Schindlbacher et al. (2009) have shown that autotrophic and heterotrophic soil respiration responded similarly to soil warming. Hence, the confounding effect of autotrophic soil respiration was supposed to be similar at control and warmed plots. As an alternative approach to estimate the metabolic activity of the bacterial community we compared bacterial RNA to DNA ratios of warmed and control plots.
2.5. Statistical analysis
We used repeated measures analysis of variance (RMANOVA) to assess warming effects on microbial biomass C, N, C/N, microbial metabolic activity and microbial stress. RMANOVA was exclusively applied to data from sampling dates, where soil was actively warmed (growing season 2008 and 2009 dates). Linear regression analysis was performed to determine if microbial biomass or specific microbial group abundances were correlated to environmental drivers such as soil temperature or soil moisture. Microbial community composition was analyzed by a principal component analysis (PCA) of the concentration of individual PLFAs. Linear regression analysis was used for relating PC scores to microbial biomass C, N, C/N, soil temperature and soil moisture. For comparing qPCR results obtained from warmed and control plots we used a Student's T-test. PCA was performed by PRIMER 6 (PRIMER-E Ltd, www.primer-e.com). All other statistical analyses were processed using SAS version 9.1 (SAS Institute, www.sas.com). The level of significance was 5% for all performed analyses.
3. Results
Warming constantly increased soil temperatures by 4 °C throughout both growing seasons and accordingly during all growing season sampling dates. During soil sampling, control plot soil temperatures varied between 14.7 °C in July and 7.2 °C and 7.9 °C in May and September 2008. Growing season soil temperatures were 11.8 °C, 13.5 °C and 12.4 °C in June, August, and September 2009. During snow-cover, soil was not warmed and soil temperatures at both control and warmed plots were slightly above freezing. Soil moisture ranged from 44.2% to 50.6% on control plots, and between 48.5% and 54.8% on warmed plots. Soil moisture at warmed and control plots did not differ significantly (RMANOVA).
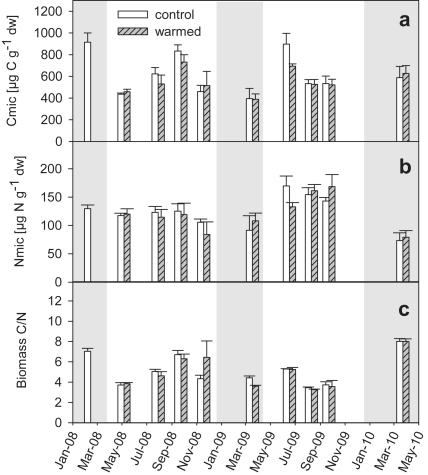
No significant soil warming effects on microbial biomass C, N, and C/N could be detected. Sampling date did not significantly affect microbial biomass C, N, and C/N either (RMANOVA). Missing seasonality in microbial biomass C and N was mirrored as insignificant relationship between microbial biomass C, N, C/N, and soil temperature. Neither did microbial biomass C, N, and C/N show any correlation with soil moisture. Fig. 1 shows microbial biomass C and N contents and C/N ratios in warmed and control plot soil during all sampling dates.
Fig. 1.
Microbial biomass C (a), N (b) and C/N (c) at control (white bars) and warmed (gray bars) plots; means ± SE (n = 3). Gray shadings indicate dormant seasons, when soil was not warmed.
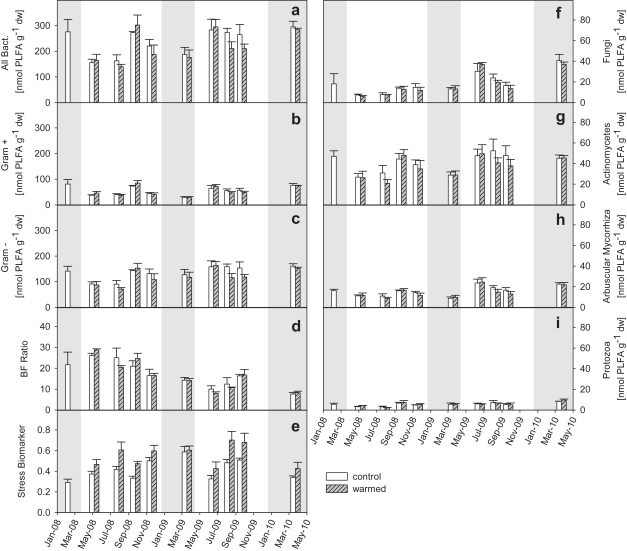
Soil warming did not affect the PLFA composition in the soil (Fig. 2). Nor did soil warming affect the abundances of the microbial groups which were separated (Fig. 3; no significant warming effect on any microbial group according to RMANOVA). Applying RMANOVA for all individual 32 PLFA peaks, only two PLFAs, the 10Me18:0 (indicator for actinomycetes; p = 0.04) and 18:1ω11 (indicator for Gram-negative bacteria; p = 0.02) were significantly lowered in warmed plots. These results were confirmed by qPCR analysis (Table 1). No warming effect was detected for the microbial groups which were analyzed by qPCR either. Mean abundances of archaeal DNA copies were lower on warmed plots during both sampling dates (Table 1). The difference was however not statistically significant in May 2008. In July 2008, the difference was almost significant (p = 0.059).
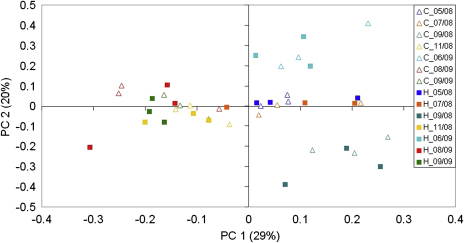
Fig. 2.
Ordination of control (C) and heated (H) subplots at different sampling dates (e.g. 05/08 = May 2008) in a score/score plot of principal component analysis conducted with 32 PLFA peaks. Explained variances are shown in parentheses for each principal component (PC).
Fig. 3.
Temporal patterns of microbial groups as separated by PLFA analysis. PLFA abundances of all bacteria (a), Gram-positive bacteria (b), Gram-negative bacteria (c), fungi (f), actinomycetes (g), arbuscular mycorrhiza (h) and protozoa (i). Temporal pattern of bacterial to fungal ratios (d) and stress biomarkers in terms of cy17:0/16:1ω7 ratios (e). Bars (white, control; gray warmed) represent plot means ± SE (n = 3). Gray shadings indicate dormant seasons, when soil was not warmed.
Table 1.
Nucleic acid yield and quantification of ribosomal gene copies by qPCR from sampling dates May and July 2008. Mean values and standard error (n = 3, in parentheses) are given.
| Nucleic acid yield (μg g DW−1) |
Bacterial 16S rRNA genes (copies ng DNA−1) |
Actinobacterial 16S rRNA genes (copies ng DNA−1) |
Fungal 28S rRNA genes (copies ng DNA−1) |
Archaeal 16S rRNA genes (copies ng DNA−1) |
Bacterial 16S rRNA RNA/DNA ratio |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Warm | Control | Warm | Control | Warm | Control | Warm | Control | Warm | Control | Warm | |
| May | 108 (42) | 115 (30) | 7.5 × 105 (4.9 × 104) | 7.2 × 105 (3.8 × 104) | 6.3 × 104 (2.5 × 103) | 7.3 × 104 (5.1 × 103) | 6.8 × 103 (1.6 × 103) | 9.6 × 103 (1.9 × 103) | 7.8 × 102 (5.0 × 102) | 5.8 × 102 (3.3 × 102) | 1.6 (0.7) | 2.7 (1.1) |
| July | 144 (31) | 118 (21) | 6.9 × 105 (2.4 × 104) | 7.0 × 105 (2.1 × 104) | 6.3 × 104 (1.3 × 103) | 6.7 × 104 (1.5 × 103) | 7.3 × 103 (1.3 × 103) | 8.7 × 103 (2.8 × 103) | 1.4 × 103 (8.6 × 102) | 3.0 × 102 (2.9 × 102) | 3.7 (2.5) | 4.7 (3.4) |
Abundances of individual microbial groups significantly varied throughout the observation timeframe (Gram-negative, p = 0.02; fungi, p < 0.001; protozoa, p = 0.04; bacteria/fungi ratios, p = 0.01 RMANOVA). Fig. 2 (PCA) shows a separation of sampling dates along the first two principal components (PC) determined by a subset of marker fatty acids from the microbial community. The respective first PC (PC1) correlated with 14 different PLFAs (eight unspecific bacterial fatty acids, two mono-unsaturated and cyclopropyl saturated PLFAs typical for Gram-negative bacteria (16:1:9, cy18:0), three terminal-branched saturated PLFAs indicative for Gram-positive bacteria (a15:0, i15:0, a16:0) and one PLFA indicative for actinomycetes (10Me18:0)). The respective second PC (PC2) correlated with six fatty acids (one typical for actinomycetes (10Me17:0), one unspecific bacterial fatty acid (16:1ω10), one marker for arbuscular mycorrhiza (16:1ω11), the indicator for fungal biomass (18:2ω6.9), the protozoan fatty acids 20:0 and 20:4ω6, and one fatty acid (i16:0) indicative for Gram-positive bacteria).
As observed for total microbial biomass, the microbial groups did not show seasonality in terms of summer/winter patterns (Fig. 3) and were not correlated to soil temperature or soil moisture. Regression analysis on PCA scores did not show any significant relationship with soil temperature, soil moisture, microbial C and microbial N. Only microbial biomass C/N ratios correlated positively with PC 1 scores (p < 0.001), indicating that community composition was related to microbial biomass C/N. Microbial biomass C/N was further positively correlated to fungal abundance during 2008 (R2 = 0.44, p = 0.048) and 2009 (R2 = 0.73, p = 0.002), when years were analyzed separately.
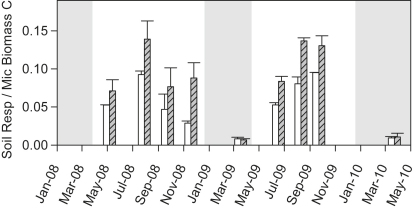
Microbial metabolic activity in terms of soil respiration per unit microbial biomass was strongly enhanced on warmed plots during most sampling dates, except winter dates, where soil was not warmed (Fig. 4). The warming effect on metabolic activity was highly significant (p < 0.001; RMANOVA). Respiration/biomass ratios on both control and warmed plots strongly correlated with soil temperature (control, R2 = 0.89, p < 0.001; warmed, R2 = 0.84, p < 0.001). Bacterial RNA/DNA ratios used as a measure of bacterial activity were higher at warmed plots (Table 1). Due to the high spatial variation, the difference was however statistically not significant.
Fig. 4.
Microbial metabolic activity expressed as field soil respiration rates per microbial biomass concentrations (means ± SE, n = 3). White bars represent control plots, gray bars warmed plots. Gray shadings indicate dormant seasons, when soil was not warmed.
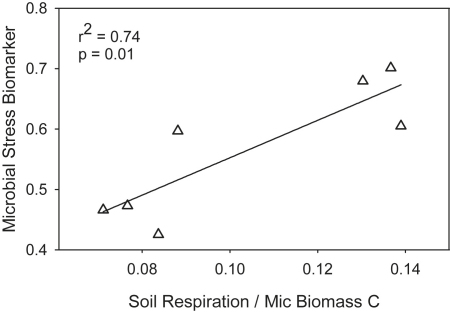
The biomarker for microbial stress was significantly (p < 0.001, RMANOVA) higher on warmed plots. During soil warming, the microbial stress biomarker was positively correlated to microbial metabolic activity (Fig. 5). The stress biomarker did not correlate with metabolic activity ratios at control plots.
Fig. 5.
Relationship between mean microbial stress biomarker (cy17:0/16:1ω7 ratios) and metabolic activity (field soil respiration rates per microbial biomass C) during soil warming in 2008 and 2009 snow-free seasons.
4. Discussion
Our results clearly show that four to five years of warming did not affect microbial biomass and community composition of the studied soil. Our hypothesis that increased soil temperatures generated advantages for specific microbial groups which better adapted to warmer conditions was not confirmed. No warming induced shifts within the microbial community composition were observed. At least not in the resolution, our methods performed. Shifts within the microbial units which were identified by qPCR or PLFA analysis; e.g. single species shifts within the fungal community, can however not be excluded.
Soil respiration rates during the sampling years 2008 and 2009 were in a similar range as at the beginning of the soil warming experiment in 2005/2006 (Schindlbacher et al., 2009). This and the similar amount of microbial biomass in control and warmed soil suggest that warming has not caused a lack in substrate availability or physiological adaptations of decomposing soil microorganisms. Furthermore, soil warming did not reduce soil moisture at our rather humid, north-exposed site. Hence, in our experiment, we had the opportunity to study the effects of elevated soil temperatures without much variation in other related parameters. “Warming effects” are often used as a relatively broad term that comprehends the effects of increased soil temperature plus all the side effects; for instance, warming induced reductions of soil moisture (e.g. Allison and Treseder, 2008; Arnold et al., 1999), gradual decline of easily available substrate (Biasi et al., 2008; Kirschbaum, 2004), or warming induced shifts in above or belowground plant biomass (Rinnan et al., 2008, 2009; Zhang et al., 2005). A closer look at results from many warming studies shows that increased soil temperature alone (the “warming” in the strict sense) might have had limited or no effects on microbial community composition.
Zhang et al. (2005), for instance, found an increase in fungal abundance after two years of ecosystem warming in a tallgrass prairie. By adding a clipping experiment, they revealed that the relative increase in fungi was likely caused by increased plant growth rather than by the increased soil temperature. By warming a field, Castro et al. (2010) found increased fungal gene copies. They also suggested indirect effects of plant community changes as an explanation of the fungal community response. While Castro et al. (2010) and Zhang et al. (2005) observed relatively fast shifts in microbial community composition, open top chamber experiments in the arctic showed that it can take more than a decade to detect first changes in the soil microbial community composition (Rinnan et al., 2007, 2009). The long time-lag between beginning of warming and first shifts in microbial community composition suggests that they were most likely driven by slowly changing aboveground biomass and plant community composition and not by elevated soil temperature alone (Rinnan et al., 2007).
Another side effect of warming which could influence the microbial community composition is the gradual depletion of easily decomposable substrate. This effect becomes most evident in lab incubations where usually no fresh substrate is supplied to the incubated soil (Feng and Simpson, 2009) and in field experiments where soil is intensively warmed over longer times (Kirschbaum, 2004; Melillo et al., 2002). In an incubation study Feng and Simpson (2009) found that PLFAs relating to fungi declined relatively to Gram-positive bacteria in soils incubated at higher temperatures. The fungal abundance decreased with incubation time and accordingly with decreasing availability of substrate. Similar observations were made by Karhu et al. (2010). Long term soil warming studies have shown that the initial increase in soil respiration rates diminished within years to a decade (Melillo et al., 2002; Strömgren, 2001). The observed decrease in fungal abundance (Frey et al., 2008) after 12 years of soil warming at Harvard forest was likely caused by decreased substrate availability rather than an adaptation to higher soil temperatures alone.
In all the examples given above, at least one parameter aside from soil temperature changed during the experimental treatment which made it difficult to distinguish if the shifts in microbial community composition resulted from adaptations to enhanced soil temperature or from somewhere else. Our results however suggest that as long as no other factors become limited or changed, increased soil temperature alone does not have much influence on major groups of the soil microbial community.
Temporal PLFA pattern showed that microbial biomass and community composition were not related to seasonal variations in soil temperature or moisture. Microbial biomass and the abundance of all distinguished groups remained high during winter, regardless of low soil temperatures. As soil moisture usually is not a limiting factor at our rather humid site, and no drought periods occurred during sampling, soil moisture did not limit microbial abundances. The temporal variations in microbial community composition may have been driven by substrate supply and hence, by interlink of aboveground biomass and belowground biomass through litter-fall, and the transport of easily decomposable C belowground into the rhizosphere (Högberg et al., 2010; Kaiser et al., 2010). Microbial biomass C/N was related to temporal patterns in PLFAs and was positively correlated to fungal PLFA abundance. Relatively low microbial biomass C/N ratios (4–8) which were in the range of typical bacterial biomass C/N ratios (3–6) (Strickland and Rousk, 2010) indicate that bacteria likely dominated the microbial community in the topsoil. The positive linear relationship between microbial biomass C/N and fungal abundance further shows that the microbial biomass C/N may provide a relatively robust rough indication of fungal and bacterial contribution to soil microbial biomass.
Our hypothesis that soil warming strongly enhanced microbial metabolic activities was confirmed by our results. This was not surprising as the decomposition of organic matter and accordingly soil respiration rates are temperature-dependent biochemical processes (Lloyd and Taylor, 1994). As microbial biomass was similar on warmed and control plots, the temperature induced increase in microbial CO2 production could have resulted from increased microbial turnover or from increased microbial maintenance respiration. As we did not measure microbial growth rates, we can only speculate if soil warming increased either of these processes. However, under similar preconditions, Zogg et al. (1997) found that only a part of the temperature induced increase in soil respiration could be explained by increased microbial growth or turnover. They suggested that changes in substrate utilization were responsible for the major part of the temperature induced increase in CO2 emission. The more likely source of the warming induced increase in CO2 emission in our study is increased microbial maintenance respiration which has been found to be positively related to soil temperature in general (Anderson and Domsch, 1985). This would further imply that soil warming had lowered the carbon use efficiency of the decomposer community.
Elevated microbial stress biomarker in warmed soil also indicates that warming rather increased microbial maintenance respiration than microbial growth because the microbial maintenance demand (respiration per biomass) increases under environmental stress (Anderson and Domsch, 2010). A positive relationship between microbial stress and metabolic activity was also shown in our study. If the increase in soil temperature was exclusively responsible for the increase in microbial stress parameters has, however, to be questioned. During soil sampling, soil temperatures have been artificially increased from between 7.2 and 14.7 °C to between 11.2 and 18.7 °C. Temperatures that actually should have been within the tolerable range of mesophilic organisms, which includes most soil microorganisms. A positive relationship between soil temperature and microbial stress indicators was observed in several soil incubation experiments (Feng and Simpson, 2009; Petersen and Klug, 1994; Zogg et al., 1997). There, elevated stress indicators were interpreted as a combined effect of temperature and temperature-induced substrate constraint. As it was unlikely that soil warming has caused a general lack in substrate availability in our study, increased microbial stress biomarker may rather reflect short term conditions, when increased soil temperatures increase microbial activity but microbes hardly can fuel their energy demand with the currently available substrate. In summary, the soil warming by 4 °C seemed sufficient enough to expose the microbes to stress, resulting in potentially reduced carbon use efficiency of the decomposers community.
5. Conclusions
Five years of intensive (+4 °C) soil warming did not affect microbial biomass nor the abundance of major microbial groups of the forest topsoil. We did not observe any adaptations of the decomposer community to warming so far. We conclude that soil respiration in temperate forest soils that contain high amounts of C, like the soil studied, will increase proportionally with an increase in temperature at least in the short to mid-term. Our data suggest that shifts in the microbial community composition, and hence adaptations which potentially mediate an increase in soil respiration, are not likely to occur until other variables (e.g. substrate, moisture) become limited or until warming alters the composition/structure of the forest stand.
Acknowledgments
The work was funded by the Austrian Science Fund, FWF (project number P19885). Many thanks to Michael Pfeffer, Brigitte Schraufstädter, Michaela Djordjevic and Kathi Keiblinger for their support in the lab and to Candy Wong for spell-check. We further thank Sabine Göttlicher for helpful comments and two anonymous reviewers for their valuable suggestions which helped to improve the manuscript.
Footnotes
Supplementary data related to this article can be found online at doi:10.1016/j.soilbio.2011.03.005.
Appendix. Supplementary material
References
- Allison S.D., Treseder K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Global Change Biology. 2008;14:2898–2909. [Google Scholar]
- Allison S.D., Wallenstein M.D., Bradford M.A. Soil-carbon response to warming dependent on microbial physiology. Nature Geoscience. 2010;3:336–340. [Google Scholar]
- Anderson J.P.E., Domsch K.H. A physiological method for the quantitative measurement of microbial biomass in soil. Soil Biology and Biochemistry. 1978;10:215–221. [Google Scholar]
- Anderson T.H., Domsch K.H. Determination of ecophysiological maintenance carbon requirements of soil microorganisms in a dormant state. Biology and Fertility of Soils. 1985;1:81–89. [Google Scholar]
- Anderson T.H., Domsch K.H. Soil microbial biomass: the eco-physiological approach. Soil Biology and Biochemistry. 2010;42:2039–2043. [Google Scholar]
- Arnold S.S., Fernandez I.J., Rustad L.E., Zibilske L.M. Microbial response of an acid forest soil to experimental soil warming. Biology and Fertility of Soils. 1999;30:239–244. [Google Scholar]
- Bååth E., Anderson T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biology and Biochemistry. 2003;35:955–963. [Google Scholar]
- Balser T.C., Wixon D.L. Investigating biological control over soil carbon temperature sensitivity. Global Change Biology. 2009;15:2935–2949. [Google Scholar]
- Bárcenas-Moreno G., Gómez-Brandón M., Rousk J., Bååth E. Adaptation of soil microbial communities to temperature: comparison of fungi and bacteria in a laboratory experiment. Global Change Biology. 2009;15:2950–2957. [Google Scholar]
- Berg M.P., Kniese J.P., Verhoef H.A. Dynamics and stratification of bacteria and fungi in the organic layers of a scots pine forest. Biology and Fertility of Soils. 1998;26:313–322. [Google Scholar]
- Biasi C., Meyer H., Rusolimova O., Hämmerle R., Kaiser C., Baranyi C., Daims H., Lashchinsky N., Barsukov P., Richter A. Initial effects of experimental warming on carbon exchange rates, plant growth and microbial dynamics of lichen-rich dwarf shrub tundra in Siberia. Plant and Soil. 2008;307:191–205. [Google Scholar]
- Biasi C., Rusalimova O., Meyer H., Kaiser C., Wanek W., Barsukov P., Junger H., Richter A. Temperature-dependent shift from labile to recalcitrant carbon sources of arctic heterotrophs. Rapid Communications in Mass Spectrometry. 2005;19:1401–1408. doi: 10.1002/rcm.1911. [DOI] [PubMed] [Google Scholar]
- Bradford M.A., Davies C.A., Frey S.D., Maddox T.R., Melillo J.M., Mohan J.E., Reynolds J.F., Treseder K.K., Wallenstein M.D. Thermal adaptation of soil microbial respiration to elevated temperature. Ecology Letters. 2008;11:1316–1327. doi: 10.1111/j.1461-0248.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- Bru D., Martin-Laurent F., Philippot L. Quantification of the detrimental effect of a single primer-template mismatch by real-time PCR using the 16S rRNA gene as an example. Applied and Environmental Microbiology. 2008;74:1660–1663. doi: 10.1128/AEM.02403-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro H.F., Classen A.T., Austin E.E., Norby R.J., Schadt C.W. Soil microbial community response to multiple experimental climate change drivers. Applied and Environmental Microbiology. 2010;76:999–1007. doi: 10.1128/AEM.02874-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P.M., Betts R.A., Jones C.D., Spall S.A., Totterdell I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature. 2000;408:184–187. doi: 10.1038/35041539. [DOI] [PubMed] [Google Scholar]
- Davidson E.A., Janssens I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–173. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- De Deyn G.D., Cornelissen J.H.C., Bardgett R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecology Letters. 2008;11:516–531. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- Feng X., Simpson M.J. Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality. Soil Biology and Biochemistry. 2009;41:804–812. [Google Scholar]
- Fierer N., Schimel J.P., Holden P.A. Variations in microbial community composition through two soil depth profiles. Soil Biology and Biochemistry. 2003;35:167–176. [Google Scholar]
- Frey S.D., Drijber R., Smith H., Melillo J.M. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biology and Biochemistry. 2008;40:2904–2907. [Google Scholar]
- Frostegård A., Bååth E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biology and Fertility of Soils. 1996;22:59–65. [Google Scholar]
- Frostegård Å, Tunlid A., Bååth E. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Applied and Environmental Microbiology. 1993;59:3605–3617. doi: 10.1128/aem.59.11.3605-3617.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegård Å, Tunlidb A., Bååth E. Microbial biomass measured as total lipid phosphate in soils of different organic content. Journal of Microbiological Methods. 1991;14:151–163. [Google Scholar]
- Griffiths R.I., Whiteley A.S., O'Donell A.G., Bailey M.J. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Applied and Environmental Microbiology. 2000;66:5488–5491. doi: 10.1128/aem.66.12.5488-5491.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl E., Pfeffer M., Donat C., Bachmann G., Zechmeister-Boltenstern S. Composition of the microbial communities in the mineral soil under different types of natural forest. Soil Biology and Biochemistry. 2005;37:661–671. [Google Scholar]
- Herman F., Smidt S., Englisch M., Gerzabek M., Haberhauer G., Jandl R., Kalina M., Zechmeister-Boltenstern S. Investigations of nitrogen fluxes and pools on a limestone site in the Alps. Environmental Science and Pollution Research. 2002;9:46–52. doi: 10.1007/BF02987478. [DOI] [PubMed] [Google Scholar]
- Högberg M.N. Discrepancies between ergosterol and the phospholipid fatty acid 18:2(6,9) as biomarkers for fungi in boreal forest soils. Soil Biology and Biochemistry. 2006;38:3431–3435. [Google Scholar]
- Högberg M.N., Briones M.J.I., Keel S.G., Metcalfe D.B., Campbell C., Midwood A.J., Thornton B., Hurry V., Linder S., Näsholm T., Högberg P. Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytologist. 2010;187:485–493. doi: 10.1111/j.1469-8137.2010.03274.x. [DOI] [PubMed] [Google Scholar]
- IPCC . Cambridge University Press; Cambridge, United Kingdom and New York, NY, USA: 2007. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. pp. 996. [Google Scholar]
- Jia Z., Conrad R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environmental Microgiology. 2009;11:1568–1671. doi: 10.1111/j.1462-2920.2009.01891.x. [DOI] [PubMed] [Google Scholar]
- Kaiser C., Koranda M., Kitzler B., Fuchslueger L., Schnecker J., Schweiger P., Rasche F., Zechmeister-Boltenstern S., Sessitsch A., Richter A. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytologist. 2010;187:843–858. doi: 10.1111/j.1469-8137.2010.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhu K., Fritze H., Tuomi M., Vanhala P., Spetz P., Kitunen V., Liski J. Temperature sensitivity of organic matter decomposition in two boreal forest soil profiles. Soil Biology and Biochemistry. 2010;42:72–82. [Google Scholar]
- Kaur A., Chaudhary A., Kaur A., Choudhary R., Kaushik R. Phospholipid fatty acid – a bioindicator of environment monitoring and assessment in soil ecosystem. Current Science. 2005;89:1103–1112. [Google Scholar]
- Keiblinger K.M., Hall E.K., Wanek W., Szukics U., Hämmerle I., Ellersdorfer G., Böck S., Strauss J., Sterflinger K., Richter A., Zechmeister-Boltenstern S. The effect of resource quantity and resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiology Ecology. 2010;73:430–440. doi: 10.1111/j.1574-6941.2010.00912.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum M.U.F. Soil respiration under prolonged soil warming: are rate reductions caused by acclimation or substrate loss? Global Change Biology. 2004;10:1870–1877. [Google Scholar]
- Knorr W., Prentice I.C., House J.I., Holland E.A. Long-term sensitivity of soil carbon turnover to warming. Nature. 2005;433:298–301. doi: 10.1038/nature03226. [DOI] [PubMed] [Google Scholar]
- Kramer C., Gleixner G. Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biology and Biochemistry. 2006;38:3267–3278. [Google Scholar]
- Lane D.J. 16S/23S rRNA sequencing. In: Stackebrandt E., Goodfellow M., editors. Nucleic Acid Techniques in Bacterial Systematics. John Wiley & Sons, Ltd.; West Sussex, United Kingdom: 1991. pp. 115–175. [Google Scholar]
- Leininger S., Urich T., Schloter M., Schwark L., Qi J., Nicol G.W., Prosser J.I., Schuster S.C., Schleper C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006;442:806–809. doi: 10.1038/nature04983. [DOI] [PubMed] [Google Scholar]
- Lipson D.A., Monson R.K., Schmidt S.K., Weintraub M.N. The trade-off between growth rate and yield in microbial communities and the consequences for under-snow soil respiration in a high elevation coniferous forest. Biogeochemistry. 2009;95:23–35. [Google Scholar]
- Liptzin D., Williams M.W., Helmig D., Seok B., Filippa G., Chowansky K., Hueber J. Process-level controls on CO2 fluxes from a seasonally snow-covered subalpine meadow soil, Niwot Ridge, Colorado. Biogeochemistry. 2009;95:151–166. [Google Scholar]
- Lloyd J., Taylor J.A. On the temperature dependence of soil respiration. Functional Ecology. 1994;8:315–323. [Google Scholar]
- Lueders T., Friedrich M. Archaeal population dynamics during sequential reduction processes in rice field soil. Applied and Environmental Microbiology. 2000;66:2732–2742. doi: 10.1128/aem.66.7.2732-2742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm G.M., López-Gutiérrez J.C., Koide R.T., Eissenstat D.M. Acclimation to temperature and temperature sensitivity of metabolism by ectomycorrhizal fungi. Global Change Biology. 2008;14:1–12. [Google Scholar]
- McMahon S.K., Williams M.A., Bottomley P.J., Myrold D. Dynamics of microbial communities during decomposition of carbon-13C labelled reygrass fractions in soil. Soil Science Society of America Journal. 2005;69:1238–1247. [Google Scholar]
- Melillo J.M., Steudler P.A., Aber J.D., Newkirk K., Lux H., Bowles F.P., Catricala C., Magill A., Ahrens T., Morrisseau S. Soil warming and carbon-cycle feedbacks to the climate system. Science. 2002;298:2173–2176. doi: 10.1126/science.1074153. [DOI] [PubMed] [Google Scholar]
- Monson R.K., Lipson D.L., Burns S.P., Turnipseed A.A., Delany A.C., Williams M.W., Schmidt S.K. Winter forest soil respiration controlled by climate and microbial community composition. Nature. 2006;439:711–714. doi: 10.1038/nature04555. [DOI] [PubMed] [Google Scholar]
- Muyzer G., de Waal E.C., Uitterlinden A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and Environmental Microbiology. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiology Ecology. 1999;29:303–310. [Google Scholar]
- Petersen S.O., Klug M.J. Effects of sieving, storage, and incubation temperature on the phospholipid fatty acid profile of a soil microbial community. Applied and Environmental Microbiology. 1994;60:2421–2430. doi: 10.1128/aem.60.7.2421-2430.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnan R., Michelsen A., Bååth E., Jonasson S. Fifteen years of climate change manipulations alter soil microbial communities in a subarctic heath ecosystem. Global Change Biology. 2007;13:28–39. [Google Scholar]
- Rinnan R., Michelsen A., Jonasson S. Effects of litter addition and warming on soil carbon, nutrient pools and microbial communities in a subarctic heath ecosystem. Applied Soil Ecology. 2008;39:271–281. [Google Scholar]
- Rinnan R., Stark S., Tolvanen A. Responses of vegetation and soil microbial communities to warming and simulated herbivory in a subarctic heath. Journal of Ecology. 2009;97:788–800. [Google Scholar]
- Schindlbacher A., Zechmeister-Boltenstern S., Glatzel G., Jandl R. Winter soil respiration from an Austrian mountain forest. Agricultural and Forest Meteorology. 2007;146:205–215. [Google Scholar]
- Schindlbacher A., Zechmeister-Boltenstern S., Jandl R. Carbon losses due to soil warming: do autotrophic and heterotrophic soil respiration respond equally? Global Change Biology. 2009;15:901–913. [Google Scholar]
- Schinner F., Öhlinger T., Kandeler E., Margesin R. Springer; Heidelberg: 1996. Methods in Soil Biology. [Google Scholar]
- Sipos R., Székely A.J., Palatinszky M., Révész S., Máraligeti K., Nikolausz M. Effect of primer mismatch, annealing temperature and PCR cycle number on 16S rRNA gene-targetting bacterial community analysis. FEMS Microbiology Ecology. 2007;60:341–350. doi: 10.1111/j.1574-6941.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Stach J.E., Maldonado L.A., Ward A.C., Goodfellow M., Bull A.T. New primers for the class actinobacteria: application to marine and terrestrial environments. Environmental Microgiology. 2003;5:828–841. doi: 10.1046/j.1462-2920.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- Strickland M.S., Rousk J. Considering fungal:bacterial dominance in soil - methods, controls, and ecosystem implications. Soil Biology and Biochemistry. 2010;42:1385–1395. [Google Scholar]
- Strömgren M. Acta Universitatis Agriculturae Sueciae; Uppsala: 2001. Soil-surface CO2 Flux and Growth in a Boreal Norway Spruce Stand: Effects of Soil Warming and Nutrition. [Google Scholar]
- Trumbore S.E., Chadwick O.A., Amundson R. Rapid exchange between soil carbon and atmospheric carbon dioxide driven by temperature change. Science. 1996;272:393–396. [Google Scholar]
- Vanhala P., Karhu K., Tuomi M., Björklöf K., Fritze H., Hyvärinen H., Liski J. Transplantation of organic surface horizons of boreal soils into warmer regions alters microbiology but not the temperature sensitivity of decomposition. Global Change Biology. 2011;17:538–550. [Google Scholar]
- White D.C., Stair J.O., Ringelberg D.B. Quantitative comparisons of in situ microbial biodiversity by signature biomarker analysis. Journal of Industrial Microbiology. 1996;17:185–196. [Google Scholar]
- Zelles L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere. 1997;35:275–294. doi: 10.1016/s0045-6535(97)00155-0. [DOI] [PubMed] [Google Scholar]
- Zhang W., Parker K.M., Luo Y., Wan S., Wallace L.L., Hu S. Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Global Change Biology. 2005;11:266–277. [Google Scholar]
- Zogg G.P., Zak D.R., Ringelberg D.B., MacDonald N.W., Pregitzer K.S., White D.C. Compositional and functional shifts in microbial communities due to soil warming. Soil Science Society of America Journal. 1997;61:475–481. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.