Figure 4.
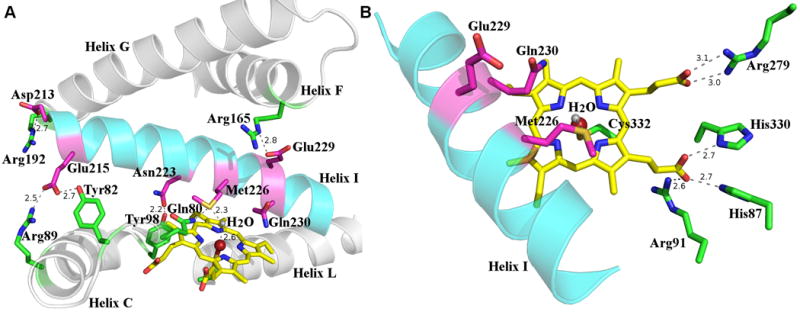
(A) Interactions between residues from I helix and those from other secondary structural elements. The I helix is shown in cyan and residues are colored in magenta, other helices are shown in gray and residues in these helices are colored in green. The heme is shown as a yellow stick, the iron shown as a maroon sphere, and the iron bound water as a gray sphere. Hydrogen bond interactions are shown as dash lines, next to which the distances are shown. (B) Active site of Orf6* showing critical catalytic residues and those interacting with the heme propionate groups. The I helix is shown in cyan, catalytically relevant residues conserved among type IV glycopeptide P450 monooxygenases are shown in magenta, Cys332 and other heme interacting residues shown in green. Hydrogen bond interactions are shown as dash lines, next to which the distances are shown.
