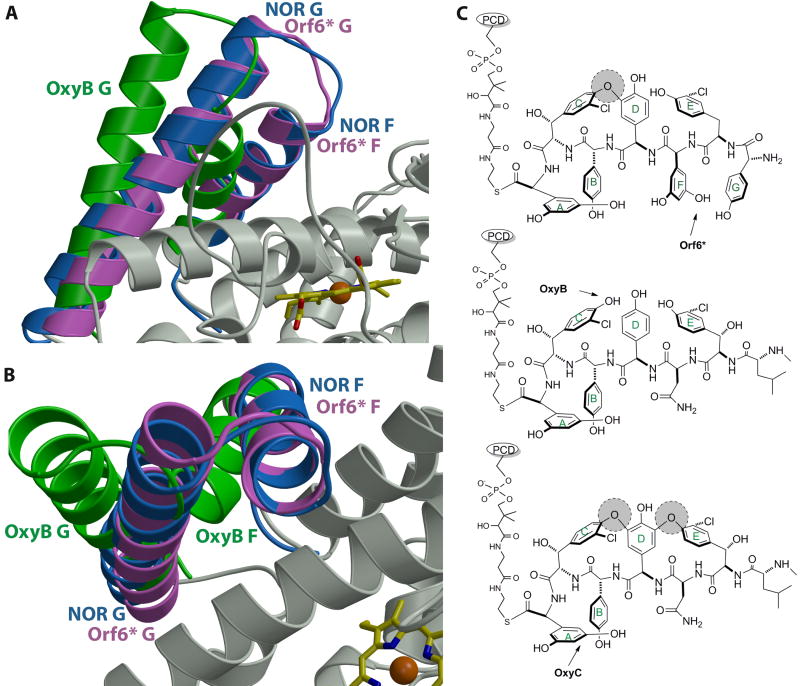
Figure 5.
(A) and (B) Orthogonal views of a least squared superposition of the crystal structure of Orf6* (in pink) with those of P450nor (in blue) and OxyB (in green). Note that the F and G helices of Orf6* and OxyB deviate significantly, despite the fact that both enzymes work on large, peptidic substrates. The F-G helices of Orf6* are in a closed conformation, similar to those observed in structures of P450s that work on small molecule substrates (e.g., P450nor). (C) A comparison of the substrates of Orf6*, OxyB and OxyC, which may provide a rationale for the positioning of the F and G helices in these enzymes. Existing cross-links in the substrate are shown as shaded circles and an arrow indicates the target site for each enzyme.