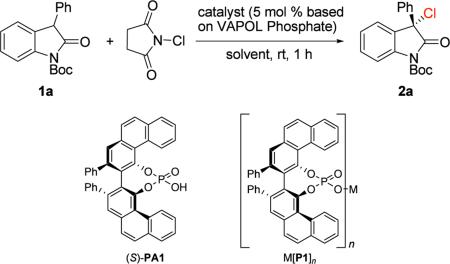
Abstract
We disclose a novel high yielding and highly enantioselective chiral calcium VAPOL phosphate-catalyzed chlorination of 3-substituted oxindoles with N-chlorosuccinimide (NCS). The reaction conditions are also shown to be effective for the catalytic enantioselective Michael addition of 3-aryloxindoles to methyl vinyl ketone.
Oxindoles bearing a tetrasubstituted chiral carbon center at the 3-position are important structural motifs found in alkaloid natural products and pharmaceuticals.1 For this reason, methods for the stereoselective synthesis of chiral 3,3'-disubstituted oxindoles are of considerable interest.1–3 Chiral oxindoles containing a heteroatom at the 3-position have found applications in medicinal chemistry.4–6 Catalytic enantioselective methods for the synthesis of biologically important chiral 3-heteroatom substituted oxindoles such as 3-fluorooxindoles,4 3-hydroxyloxindoles3a,5 and 3-aminooxindoles3b,6 have been recently reported. In spite of these advancements, methods detailing the efficient enantioselective synthesis of 3-chlorooxindoles, which are potentially important in medicinal chemistry, are unavailable.7–9
Despite the fact that numerous important pharmaceutical agents contain chiral centers with chloro-substitution10 there has been little corresponding progress in asymmetric chlorination. The examples of highly enantioselective chlorination are rare and often limited to 1,3-dicarbonyl compounds, such as β-keto esters11 and aliphatic aldehydes.12 Thus, the development of novel asymmetric chlorinations involving other substrate classes is of considerable importance.
Group 2 alkaline-earth metals, such as calcium, strontium and barium are abundant, inexpensive and relatively nontoxic, but their use in organic synthesis is fairly limited.13 Applications of these metals as catalysts in asymmetric transformations is even more scarce.14 Herein, we describe a novel enantioselective chlorination of oxindoles with N-chlorosuccinimide (NCS) catalyzed by a chiral calcium VAPOL phosphate to afford the product in quantitative yield with high enantioselectivity. Furthermore, we demonstrate that the methodology can be effectively extended to the catalytic enantioselective Michael addition of oxindoles to methyl vinyl ketone.
As part of our ongoing program toward the development of new chiral phosphoric acid-catalyzed asymmetric reactions,15 we theorized that chiral phosphoric acids could activate NCS via protonation of the imide carbonyl. Following an initial screening of chiral phosphoric acids (purified by silica gel column chromatography),16 we found VAPOL phosphoric acid (PA1) to be the best catalyst in terms of enantioselectivity. PA1 also demonstrated the ability to dramatically accelerate the reaction (Table 1, entry 2 vs. entry 1). Solvent screening showed isopropyl acetate to be the most suitable solvent, allowing for 80% ee of 2 in just 1 h (Table 1, entries 2–6). When the concentration was decreased to 0.05 M, the ee increased to 90% (Table 1, entry 7).
Table 1.
Catalyst Optimization for the Asymmetric Chlorination of 3-Phenyloxindole 1a.a
| entry | catalyst | solvent | yield (%)b | ee (%)c |
|---|---|---|---|---|
| 1d | - | toluene | < 20 | - |
| 2 | PA1 purified on silica gel | toluene | 99 | 51 |
| 3 | PA1 purified on silica gel | DCM | 99 | 48 |
| 4 | PA1 purified on silica gel | EtOAc | 99 | 50 |
| 5 | PA1 purified on silica gel | benzene | 99 | 60 |
| 6 | PA1 purified on silica gel | i-PrOAc | 99 | 80 |
| 7e | PA1 purified on silica gel | i-PrOAc | 99 | 90 |
| 7e,f | PA1 washed with HCI | i-PrOAc | 99 | 0 |
| 9e | Na[P1] | i-PrOAc | 99 | 6 |
| 10e | K[P1] | i-PrOAc | 99 | 0 |
| 11e | Mg[P1]2 | i-PrOAc | 99 | 37 |
| 12e | Ca[P1]2 | i-PrOAc | 99 | 91 |
| 13e | Sr[P1]2 | i-PrOAc | 99 | 86 |
| 14e | Ba[P1]2 | i-PrOAc | 99 | 9 |
| 15g | Ca[P1]2 | i-PrOAc | 99 | 94 |
Reaction conditions: 1a (1.0 equiv), NCS (1.2 equiv), 5 mol % catalyst (based on VAPOL phosphate), with solvent indicated [0.10 M].
Isolated yield.
Enantiomeric excess determined by chiral HPLC analysis.
Reaction time of 24 h.
Reaction performed at [0.050 M].
Reaction time of 3 h.
NCS was added as a [0.12 M] solution in i-PrOAc.
Surprisingly, PA1 washed with 6 N HCl, led to the formation of racemic product (Table 1, entry 8). Comparison of this result to a recent report by Ishihara and co-workers,17 documenting the presence of chiral phosphate salts in the absence of a final HCl wash of the chiral phosphoric acid/salt mixture obtained by silica gel purification, led us to investigate different chiral VAPOL phosphate salts (Table 1, entries 9–14). Sodium and K derived VAPOL phosphates afforded racemic product (Table 1, entries 9 and 10). Magnesium, Ca, and Sr derived VAPOL phosphates allowed for the chlorination product with dramatically increased enantioselectivity (Table 1, entries 11–14). To our delight, the calcium derived VAPOL phosphate salt was able to yield the product with 99% yield and 91% ee. The enantioselectivity could be further increased to 94% through the slow addition of NCS.18
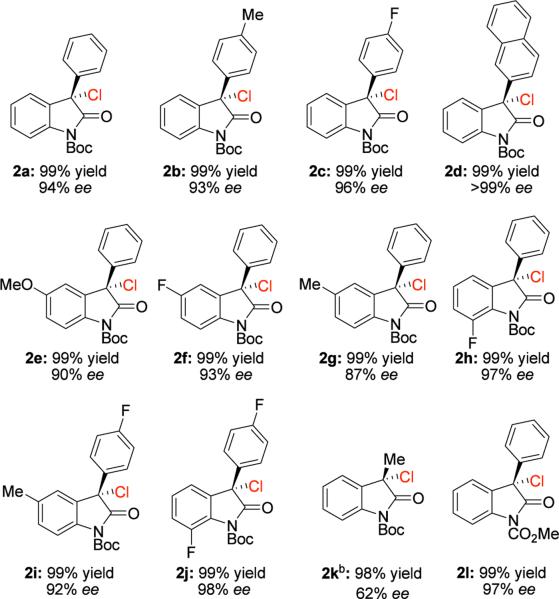
With the optimized conditions in hand, we turned our focus to the substrate scope and generality of the reaction. The introduction of electron-donating and electron-withdrawing groups on both the oxindole core and the 3-aryl group provided for products with excellent enantioselectivity with 10 minute reaction times. The enantioselectivity of products bearing electron-donating groups was found to be slightly lower than that of products bearing electron-withdrawing groups (Table 2, 2b vs. 2c and 2e vs. 2f). High yield and modest enantioselectivity can be obtained even with an alkyl substituent at the 3 position of the oxindole. With a 3-methyl substituted oxindole as a substrate, the reaction affords the product with 98% yield and 62% ee (Table 2, 2k), although a longer reaction time is required. Variation of the carbamate protecting group afforded the product with high enantioselectivity (Table 2, 2l), while lower ee or complete loss of reactivity was observed with other protecting groups on nitrogen.19
Table 2.
Substrate Scope for the Ca[P1]2 Catalyzed Asymmetric Chlorination.a

|
Reaction conditions: 1 (1.0 equiv), Ca[P1]2 (2.5 mol %), and NCS (1.2 equiv) is added as a 0.12 M solution in i-PrOAc over 20 min. The (S)-VAPOL derived salt was used in each example.
Reaction time of 80 min.
Although a detailed mechanism has yet to be elucidated, we propose a transition-state (Figure 1) that highlights the bifunctional nature of the chiral calcium VAPOL phosphate in activation of both the nucleophile and the electrophile.14,17 Chelation of the carbonyl oxygens of the BOC group and the NCS to calcium creates a more compact reaction sphere. The increased Brønsted basicity of the chiral phosphate further activates the oxindole tautomer. Hydrogen bonding interactions between the oxindole tautomer and NCS coupled with the above mentioned interactions could allow for the high enantiocontrol observed.
Figure 1.
Proposed transition-state for the asymmetric chlorination of oxindole 1a.
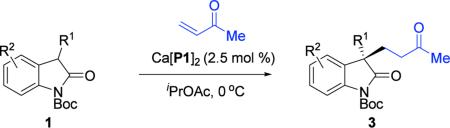
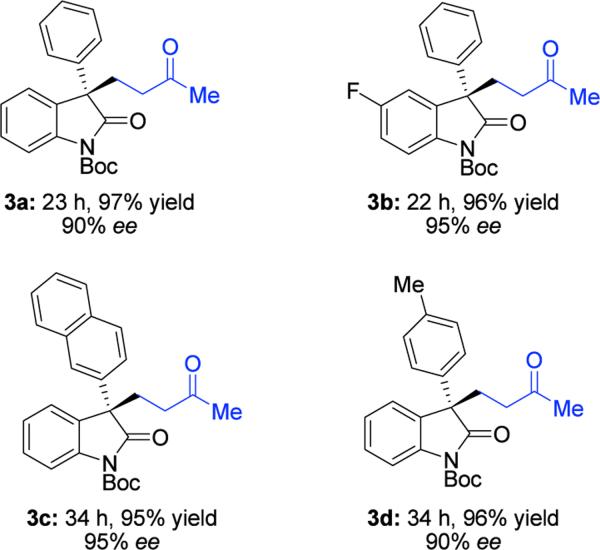
Based on the proposed transition-state, we envisioned this newly developed chlorination protocol could be applied to other reaction systems. Methyl vinyl ketone (MVK) is an exemplary electrophile for Michael additions.3e,20 Application of our chlorination protocol to MVK afforded products bearing a quaternary carbon center with both high yield and high enantioselectivity (Table 3).21
Table 3.
Asymmetric Michael Reaction of Oxindoles and Methyl Vinyl Ketone Catalyzed by Ca[P1]2a
|
Reaction conditions: 1 (1.0 equiv), methyl vinyl ketone (3.0 equiv), and 2.5 mol % catalyst at 0 °C. Yields reported are isolated. Enantiomeric excess was determined by Chiral HPLC Analysis. The (R)-VAPOL derived salt was used in each example.
In conclusion, we have developed a facile method for the highly efficient enantioselective chlorination of oxindoles catalyzed by a novel chiral calcium VAPOL phosphate salt. This method provides access to 3-chloro-oxindole products with high enantioselectivity. The aforementioned VAPOL phosphate salt was also found to be a highly effective promoter for the Michael reaction of 3-aryloxindoles with methyl vinyl ketone. Mechanistic investigations and extension of the asymmetric chlorination protocol to additional reaction systems is currently under investigation in our laboratory and will be reported in due course.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health (NIH GM-082935) and the National Science Foundation CAREER Program (NSF-0847108) for financial support.
Footnotes
Supporting Information Available: Experimental procedures and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org
References
- (1).For Reviews, see: Galliford CV, Scheidt KA. Angew. Chem., Int. Ed. 2007;46:8748. doi: 10.1002/anie.200701342. Dounay AB, Overman LE. Chem. Rev. 2003;103:2945. doi: 10.1021/cr020039h.
- (2).For selected reviews, see: Zhou F, Liu Y-L, Zhou J. Adv. Synth. Catal. 2010;352:1381. Trost BM, Brennan MK. Synthesis. 2009:3003. Lin H, Danishefsky SJ. Angew. Chem., Int. Ed. 2003;42:36. doi: 10.1002/anie.200390048. Marti C, Carreira EM. Eur. J. Org. Chem. 2003:2209.
- (3).Catalytic asymmetric syntheses of 3,3'-disubstituted oxindoles: Bui T, Candeias NR, Barbas CF., III J. Am. Chem. Soc. 2010;132:5574. doi: 10.1021/ja101032j. Mouri S, Chen Z, Mitsunuma H, Furutachi M, Matsunaga S, Shibasaki M. J. Am. Chem. Soc. 2010;132:1255. doi: 10.1021/ja908906n. Taylor AM, Altman RA, Buchwald SL. J. Am. Chem. Soc. 2009;131:9900. doi: 10.1021/ja903880q. He R, Shirakawa S, Maruoka K. J. Am. Chem. Soc. 2009;131:16620. doi: 10.1021/ja906821y. He R, Ding C, Maruoka K. Angew. Chem., Int. Ed. 2009;48:4559. doi: 10.1002/anie.200901277. Kato Y, Furutachi M, Chen Z, Mitsunuma H, Matsunaga S, Shibasaki M. J. Am. Chem. Soc. 2009;131:9168. doi: 10.1021/ja903566u. Duffey TA, Shaw SA, Vedejs E. J. Am. Chem. Soc. 2009;131:14. doi: 10.1021/ja805541u. Linton EC, Kozlowski MC. J. Am. Chem. Soc. 2008;130:16162. doi: 10.1021/ja807026z. Trost BM, Cramer N, Silverman SM. J. Am. Chem. Soc. 2007;129:12396. doi: 10.1021/ja075335w. Corkey BK, Toste FD. J. Am. Chem. Soc. 2007;129:2764. doi: 10.1021/ja068723r. Poulsen TB, Bernardi L, Alemán J, Overgaard J, Jørgensen KA. J. Am. Chem. Soc. 2007;129:441. doi: 10.1021/ja067289q. Kűndig EP, Seidel TM, Jia Y-X, Bernardineli G. Angew. Chem., Int. Ed. 2007;46:8484. doi: 10.1002/anie.200703408. Hills ID, Fu GC. Angew. Chem., Int. Ed. 2003;42:3291. doi: 10.1002/anie.200351666.
- (4).(a) Ishimaru T, Shibata N, Horikawa T, Yasuda N, Nakamura S, Toru T, Shiro M. Angew. Chem., Int. Ed. 2008;47:4157. doi: 10.1002/anie.200800717. [DOI] [PubMed] [Google Scholar]; (b) Shibata N, Kohno J, Takai K, Nakamura S, Toru T, Kagemasa S. Angew. Chem., Int. Ed. 2005;44:4204. doi: 10.1002/anie.200501041. [DOI] [PubMed] [Google Scholar]; (c) Hamashima Y, Suzuki T, Takano H, Shimura Y, Sodeoka M. J. Am. Chem. Soc. 2005;127:10164. doi: 10.1021/ja0513077. [DOI] [PubMed] [Google Scholar]
- (5).(a) Liu Y-L, Wang B-L, Cao J-J, Chen L, Zhang C, Wang Y-X, Zhou J. J. Am. Chem. Soc. 2010;132 doi: 10.1021/ja107858z. DOI: 10.1021/ja107858z. [DOI] [PubMed] [Google Scholar]; (b) Itoh T, Ishikawa H, Hayashi Y. Org. Lett. 2009;11:3854. doi: 10.1021/ol901432a. [DOI] [PubMed] [Google Scholar]; (c) Tomita D, Yamatsugu K, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2009;131:6946. doi: 10.1021/ja901995a. [DOI] [PubMed] [Google Scholar]; (d) Itoh J, Han SB, Krische MJ. Angew. Chem., Int, Ed. 2009;48:6313. doi: 10.1002/anie.200902328. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Shintani R, Inoue M, Hayashi T. Angew. Chem., Int. Ed. 2006;45:3353. doi: 10.1002/anie.200600392. [DOI] [PubMed] [Google Scholar]; (f) Ishimaru T, Shibata N, Nagai J, Nakamura S, Toru T, Kanemasa S. J. Am. Chem. Soc. 2006;128:16488. doi: 10.1021/ja0668825. [DOI] [PubMed] [Google Scholar]
- (6).(a) Bui T, Borregan M, Barbas CF., III J. Org. Chem. 2009;74:8935. doi: 10.1021/jo902039a. [DOI] [PubMed] [Google Scholar]; (b) Cheng L, Liu L, Wang D, Chen Y-J. Org. Lett. 2009;11:3874. doi: 10.1021/ol901405r. [DOI] [PubMed] [Google Scholar]; (c) Qian Z-Q, Zhou F, Du T-P, Wang B-L, Zhou J. Chem. Commun. 2009:6753. doi: 10.1039/b915257a. [DOI] [PubMed] [Google Scholar]
- (7).See ref 4b for a single example with a 61% ee.
- (8).Gribkoff WK, Post-Munson DJ, Yeola SW, Boissard CG, Hwwawasam P. WO 2002030868.
- (9).For transformation of 3-chlorooxindole to other oxindole compounds, see: Ma S, Han X, Krishnan S, Virgil SC, Stoltz BM. Angew. Chem., Int. Ed. 2009;48:8037. doi: 10.1002/anie.200902943. Grant CD, Krische MJ. Org. Lett. 2009;11:4485. doi: 10.1021/ol9018562.
- (10).For selected reviews, see: Oestreich M. Angew. Chem., Int. Ed. 2005;44:2324. doi: 10.1002/anie.200500478. Ibrahim H, Togni A. Chem. Commun. 2004:1147.
- (11).(a) Cai Y, Wang W, Shen K, Wang J, Hu X, Lin L, Liu X, Feng X. Chem. Commun. 2010:1250. doi: 10.1039/b922769e. [DOI] [PubMed] [Google Scholar]; (b) Bartoli G, Bosco M, Carlone A, Locatelli M, Melchiorre P, Sambri L. Angew. Chem., Int. Ed. 2005;44:6219. doi: 10.1002/anie.200502134. [DOI] [PubMed] [Google Scholar]; (c) Marigo M, Kumaragurubaran N, Jørgensen KA. Chem. Eur. J. 2004;10:2133. doi: 10.1002/chem.200305759. [DOI] [PubMed] [Google Scholar]; (d) Hintermann L, Togni A. Helv. Chim. Acta. 2000;83:2425. [Google Scholar]
- (12).(a) Amatore M, Beeson TD, Brown SP, MacMillan DWC. Angew. Chem., Int. Ed. 2009;48:5121. doi: 10.1002/anie.200901855. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brochu MP, Brown SP, MacMillan DWC. J. Am. Chem. Soc. 2004;126:4108. doi: 10.1021/ja049562z. [DOI] [PubMed] [Google Scholar]; (c) Holland N, Braunton A, Bachmann S, Marigo M, Jørgensen KA. Angew. Chem., Int. Ed. 2004;43:5507. doi: 10.1002/anie.200460462. [DOI] [PubMed] [Google Scholar]; (d) Zhang Y, Shibatomi K, Yamamoto H. J. Am. Chem. Soc. 2004;126:15038. doi: 10.1021/ja0454485. [DOI] [PubMed] [Google Scholar]
- (13).Cotton FA, Wilkinson G, Gaus PL, editors. Basic Inorganic Chemistry. 3rd ed. Wiley; New York: 1995. [Google Scholar]
- (14).Selected Ca-catalyzed asymmetric reactions: Tsubogo T, Saito S, Seki K, Yamashita Y, Kobayashi S. J. Am. Chem. Soc. 2008;130:13321. doi: 10.1021/ja8032058. Kumaraswamy G, Jena N, Sastry MNV, Padmaja M, Markondaiah B. Adv. Synth. Catal. 2005;347:867. Suzuki T, Yamagiwa N, Matsuo Y, Sakamoto S, Yamaguchi K, Shibasaki M, Noyori R. Tetrahedron Lett. 2001;42:4669. Sr catalysts: Agostinho M, Kobayashi S. J. Am. Chem. Soc. 2008;130:2430. doi: 10.1021/ja710332h. Ba catalysts: Saito S, Kobayashi S. J. Am. Chem. Soc. 2006;128:8704. doi: 10.1021/ja061221t. Yamatsugu K, Yin L, Kamijo S, Kimura Y, Kanai M, Shibasaki M. Angew. Chem., Int. Ed. 2009;48:1070. doi: 10.1002/anie.200804777.
- (15).For reviews, see: Terada M. Synthesis. 2010:1929. Terada M. Chem. Commun. 2008:4097. doi: 10.1039/b807577h. Akiyama T. Chem. Rev. 2007;107:5744. doi: 10.1021/cr068374j.
- (16).For details on our initial catalyst/solvent screening, please see the supporting information.
- (17).Hatano M, Moriyama K, Maki T, Ishihara K. Angew. Chem., Int. Ed. 2010;49:3823. doi: 10.1002/anie.201000824. [DOI] [PubMed] [Google Scholar]
- (18).When the reaction was run under identical conditions with NBS as the electrophile a 48% ee was found for the brominated product.
- (19).60% ee was observed for the Ac-protected oxindole, while no reactivity was seen for the Bn-protected oxindole. The Ts-protected product was unable to be separated by HPLC.
- (20).(a) Akiyama T, Katoh T, Mori K. Angew. Chem., Int. Ed. 2009;48:4226. doi: 10.1002/anie.200901127. [DOI] [PubMed] [Google Scholar]; (b) Wu F, Li H, Hong R, Deng L. Angew. Chem., Int. Ed. 2006;45:947. doi: 10.1002/anie.200502658. [DOI] [PubMed] [Google Scholar]
- (21).Examples of additional Michael acceptors that were tested can be found in the supporting information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



