Pyogenic granuloma is a benign, lobular capillary hemangioma that most commonly occurs on the skin. It also has been occasionally found on the mucosal surfaces of the oral cavity and the upper respiratory tract. Only a handful of cases, most of which come from the Japanese literature, have been reported in the gastrointestinal tract, predominantly in the esophagus and intestine. We report here a rare case of gastric pyogenic granuloma associated with chronic bleeding and iron deficiency anemia and present its endoscopic and pathologic findings. We also report and discuss the use of endoscopic ultrasound in the evaluation of the endoscopic resectability of these lesions. Finally, we review the English, Japanese, and French literature on gastrointestinal pyogenic granulomas.
Case Report
A 67-year-old woman presented to the emergency room with complaints of shortness of breath and chest pains suggestive of angina. Medical evaluation revealed microcytic hypochromic anemia (hematocrit of 24%) due to iron deficiency (serum iron of 19µg/dL, transferrin of 485 µg/dL, transferrin saturation of 4%, and ferritin of 6.6 ng/mL). The patient received a transfusion of packed red blood cells, which led to the immediate resolution of her symptoms, and she was subsequently started on oral ferrous sulfate therapy. A recent screening colonoscopy had been reported to be unremarkable. The patient was therefore scheduled for an esophagogastroduodenoscopy (EGD).
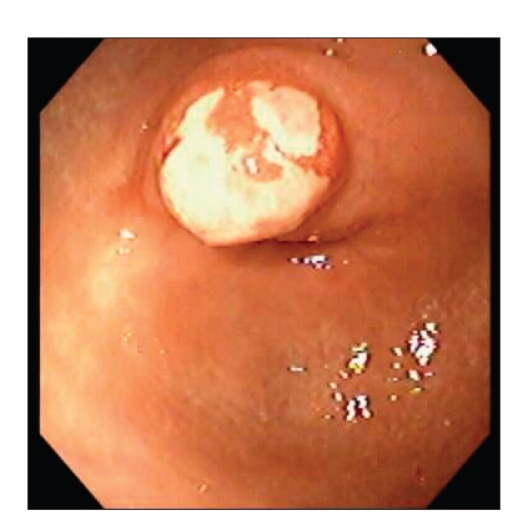
EGD revealed a 10-mm sessile polypoid lesion with a short broad stalk in the gastric antrum. The surface of the lesion had a variegated appearance with yellow ulcerated areas overlying a reddish/erythematous background (Figure 1). The adjacent antral mucosa was mildly erythematous but otherwise normal. No other potential sources of blood loss were evident in the upper gastrointestinal tract. Biopsies of the lesion obtained at EGD revealed nonspecific appearances, with acute inflammation, ulceration, and granulation tissue. Biopsies from the adjacent antral mucosa were unremarkable, with no evidence of gastritis or Helicobacter pylori.
Figure 1.
Polypoid antral lesion.
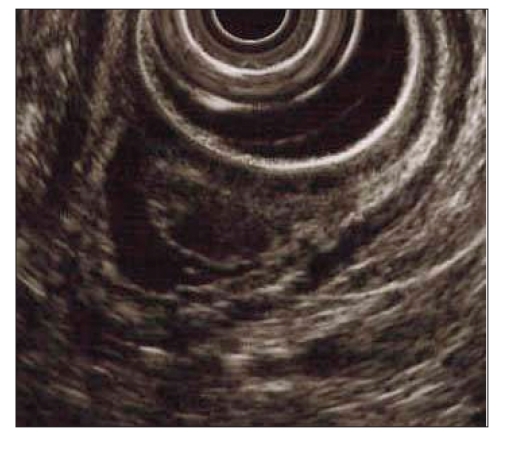
An endoscopic ultrasound (EUS) was subsequently performed using Olympus radial and linear echoendoscopes. EUS confirmed that the lesion was confined to the mucosa. No large blood vessels or vascular spaces were noted within the lesion (Figure 2).
Figure 2.
Endoscopic ultrasound image of pyogenic granuloma.
The polypoid mucosal lesion was then elevated with a submucosal injection of 12 mL of 1:100,000 epinephrine solution using a sclerotherapy needle. The lesion was then resected using a polypectomy snare and retrieved for pathology. No bleeding was noted following endoscopic resection of the lesion.
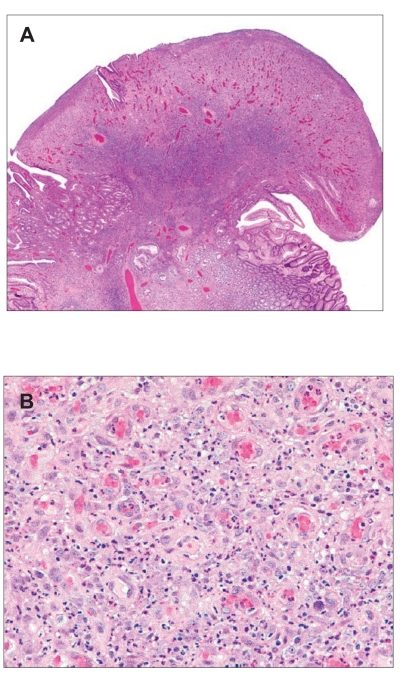
On pathologic analysis, the excised lesion was noted to be a discrete, exophytic, polypoid mass of granulation tissue with a lobular proliferation of capillary-sized vessels with intermixed acute and chronic inflammatory cells (Figure 3). Residual gastric glands formed collarettes along the sides of the vascular tissue proliferation. A Warthin-Starry stain for bacillary organisms tested negative. The histopathologic diagnosis was concluded to be pyogenic granuloma of the stomach.
Figure 3.
Histology of pyogenic granuloma. Resection of the polyp at esophagogastroduodenoscopy revealed surface ulceration, acute inflammation, and lobular proliferation of capillary-sized vessels with a collarette of intact mucosa along the base. Figure 3B reveals details of the capillary structures, stromal edema, sparse lymphocytes, and marked neutrophilic infiltrate.
Discussion
Pyogenic granuloma is a polypoid form of capillary hemangioma, reported most commonly on the skin and in the oral and nasal mucosa. Cutaneous forms of the lesion were initially described by Poncet and Dor in 1897,1 although the term “pyogenic granuloma” was first applied by Hartzell in 1904.2 The first clear reports of gastrointestinal pyogenic granulomas were attributed to Payson and associates in 1967.3 A small number of additional gastrointestinal lesions have since been noted, predominantly in the colon, ileum, and esophagus. Our case report is the first description of a gastric pyogenic granuloma outside of Asia.
Gastrointestinal pyogenic granulomas in the adult literature have been reported in subjects ranging from 31 to 71 years of age (median age=56 years). Whereas cutaneous lesions occur equally in both sexes, among the small number of reported gastrointestinal cases, there appears to be a male preponderance, with a male-to-female ratio of approximately 2:1. The lesions typically appear as smooth, protruding, polypoid, reddish-colored nodules that may ulcerate. The reddish color is due to the vascular nature of the lesions. Esophageal lesions have been reported as having a superficial white coating of necrotic tissue.4 On occasion, in patients presenting with significant gastrointestinal bleeding, the surface of these lesions has been noted to be necrotic and black.5 Gastrointestinal lesions are typically pedunculated or semipedunculated and are less frequently sessile. The reported sizes of gastrointestinal lesions have ranged from 5 to 750 mm in diameter. Larger lesions are more likely to show surface ulceration.6 Surface ulceration is therefore believed to be a secondary event, rather than the event inciting development of the lesion.5 Areas of infarction have been described in large lesions.3 The lesions typically involve the mucosa but may extend to the submucosa7 or, on occasion, involve the full thickness of the luminal wall, with protrusion into both luminal and serosal surfaces of the gut wall.5
Although gastrointestinal lesions are typically solitary, multiple (>10) gastrointestinal lesions have been reported in a single patient.8 These lesions were detected in the sigmoid colon of a patient presenting with diarrhea and hemoccult-positive stool. In another patient, presumed disseminated disease was reported.9 This patient was a 73-year-old man with biopsy-confirmed cutaneous lesions of pyogenic granuloma. He subsequently developed symptoms of intermittent melena with associated anemia. 99Tcm-labeled red blood cell scintigraphy revealed multiple areas of increased uptake in his skull, abdominal wall, intestine, scrotum, and right leg. The authors attributed the increased areas of intestinal uptake to intestinal pyogenic granulomas, to which they also attributed his symptoms of melena. However, the nature of the areas of increased intestinal uptake were not confirmed endoscopically or histologically. In addition, the patient had a past history of endoscopically confirmed duodenal ulceration, which might have explained his symptoms of intermittent melena.
Clinical presentation of the gastrointestinal lesions depends upon their site (Table 1). Esophageal lesions present with chest discomfort,10 dysphagia,7,11,12 odynophagia,4 hematemesis,7 or may be incidentally discovered.4 A patient with a single gastric cardia lesion previously reported in the Japanese literature presented with upper abdominal discomfort, whereas a different patient with gastric pyogenic granulomas presented with chronic melena.13 Our gastric pyogenic granuloma patient presented with iron deficiency anemia, presumably due to intermittent bleeding from this vascular lesion. Duodenal lesions have presented with anemia unresponsive to iron therapy6 or with overt upper gastrointestinal bleeding.14 Small-bowel lesions have presented with melena, anemia,5,15 and intussusception.3 Colonic lesions have presented with melena,5 hematochezia,16 and diarrhea.8 A single large circumferential stenosing colonic lesion mimicking colon cancer also has been reported.17 A different single report notes a polyp-like intestinal lesion in a patient with Peutz-Jeghers syndrome. In this series of 75 polyps excised from seven patients with Peutz-Jeghers syndrome, a single “polyp” was determined to be a pyogenic granuloma.18
Table 1.
Prior Reports of Gastrointestinal Pyogenic Granulomas
| Reference | Sex, age | Location | Presentation | Lesion # (size) | Management |
|---|---|---|---|---|---|
| English-language literature | |||||
| Okada, et al.10 | M, 56 y | Esophagus | Chest discomfort | 1 (NA) | EMR |
| Craig, et al.11 | M, 31 y | Esophagus | Dysphagia | 1 (2.5 cm) | Snare polypectomy |
| Cho, et al.22 | M, 60 y | Esophagus | Esophageal tumor | 1 (0.5 cm) | Snare polypectomy |
| van Eeden, et al.7 | • F, 55 y • F, 55 y |
• Esophagus • Small bowel |
• Hematemesis/dysphagia • Anemia/melena |
• 1 (0.9 cm) • 1 (1.1 cm) |
• Polypectomy and laser photocoagulation • Surgical resection |
| Hirakawa, et al.6 | M, 60 y | Duodenum | Anemia | 1 (0.8 cm) | Heater probe: unsuccessful; then snare polypectomy |
| Meuwissen, et al.21 | M, 37 y | Ileum (stoma) | Bleeding | NA | Surgical resection |
| Payson, et al.3 | M, 45 y | Ileum | Intussusception | 1 (7.5 cm) | Surgical resection |
| Yao, et al.5 | • M, 56 y • F, 71 y • M, 55 y |
• Jejunum • Ileum • Colon |
• Anemia/melena • Anemia • Melena |
1 (2.0–2.5 cm) | Surgical resection |
| Hocke, et al.17 | F, 60 y | Hepatic flexure | Suspected colon cancer | 1 (NA) | Hemicolectomy |
| Chen, et al.8 | M, 36 y | Sigmoid | Diarrhea, guaiac-positive | Multiple,>10 (0.4–0.8 cm) | Snare polypectomy |
| Hizawa, et al.18 | NA | NA | Peutz-Jeghers | 1 | Snare polypectomy |
| Gonzalez-Vela, et al.16 | F, 62y | Sigmoid | Hematochezia | 1 (2.0 cm) | Snare polypectomy |
| Kusakabe, et al.13 | M, 82y | Stomach | Melena | 1 (3.0 cm) | EMR |
| Non–English-language literature | |||||
| Yamane, et al25 | M, 64 y | Esophagus | NA | 1 (NA) | NA |
| Imawari, et al.4 | F, 70 y | Esophagus | Odynophagia | 1 (0.4–0.5 cm) | Snare polypectomy |
| Manabe, et al.2 | M, 45 y | Esophagus | Dysphagia | 1 (1.5 cm) | Snare polypectomy |
| Okumura, et al.24 | NA | Esophagus | NA | NA | Band ligation |
| Kogawa, et al.23 | M,50 y | Stomach | RUQ pain | 1 (0.8 cm) | EMR |
| Viala, et al.14 | M, 66 y | Duodenal bulb | UGI bleeding | 1 (NA) | Laser ablation |
| Motohashi, et al.15 | F, 58 y | Small intestine | UGI bleeding | 1 (3.0 cm) | Surgical resection |
- EMR
endoscopic mucosal resection
- RUQ
right upper quadrant
- UGI
upper gastrointestinal.
Pathologic examination of the lesions usually reveals a proliferation of dilated capillaries with a lobular arrangement. Endothelial cell swelling may also be seen. Stromal tissue is usually edematous, with an infiltration of inflammatory cells. Typically, the infiltrate is predominantly neutrophilic near the ulcerated surface of the lesion, although chronic inflammatory cells may be seen in deeper areas of the lesion. Surface ulceration and granulation tissue are frequently visible. Immunostaining for factor VIII-related antigen and CD34 has been used to identify endothelial cells in these lesions. Immunostaining with antibodies against human herpes virus 8 has been used to distinguish the lesions from gastrointestinal Kaposi sarcoma.7
Endoscopically obtained biopsies may not allow a firm diagnosis to be reached, as although they may show some combination of inflammation, granulation tissue, and ulceration, the lobular capillary arrangement may only be evident on pathology sections of the entirely excised specimen. Biopsies may also carry the theoretical risk of initiating bleeding from these vascular lesions. However, biopsy may allow other pathologic entities to be excluded.
The etiology of pyogenic granuloma is unclear. The lesions were initially thought to be infective in origin; however, no association with infection has been proven. It has been proposed that the lesions may be reactive and caused by minor trauma, with a subsequent overgrowth of granulation tissue. The occurrence of cutaneous lesions found predominantly on exposed skin surfaces, including the hands, face, and lips, has lent some support to this theory. However, a large retrospective study of pyogenic granulomas in children found clear evidence of trauma in only 5% of patients.19 Hormonal influences may play a role, as dermal and oral lesions have been reported in up to 2% of pregnancies.20 Similarly, a role has been proposed for angiogenic factors, as cutaneous pyogenic granuloma has been reported to develop within preexisting vascular malformations such as hemangiomas, spider angiomas, and port wine stains.19
Several patients have undergone baseline endoscopic evaluation prior to the diagnosis of gastrointestinal pyogenic granuloma at a subsequent endoscopic evaluation. Review of these cases does suggest that gastrointestinal pyogenic granulomas may originate at sites of prior inflammation or ulceration. One patient developed an esophageal pyogenic granuloma at a site of documented esophagitis with stricturing and was treated over several years with proton pump inhibitor therapy and endoscopic dilation.11 A duodenal pyogenic granuloma was reported in another patient with a duodenal ulcer that had been endoscopically documented 10 months prior.14 Angiomatous proliferations with some features of pyogenic granuloma were reported at an ileal stoma in a patient who had documented Campylobacter jejuni gastroenteritis with ulceration at the stoma two months prior.21
Gastrointestinal lesions have been most commonly managed by excision using a polypectomy snare,4,6–8,11,12,16,18,22 endoscopic mucosal resection,10,13,23 or surgical resection.3,5,7,15,17,21 Other modes of management have included heater probe coagulation,6 laser photocoagulation,7,14 intralesional injection of ethanol, band ligation,24 and excision biopsy.
The optimal management of gastrointestinal lesions is by complete excision, preferably with snare resection or endoscopic mucosal resection. Management by other techniques such as heater probe coagulation and excision biopsy have been associated with persistence or recurrence of the lesion.6,24 In addition, these incomplete therapies may trigger further bleeding. Hirakawa and colleagues initially treated an anemic patient with a duodenal lesion via heater probe.6 Three months later, the patient was readmitted with worsening anemia. The patient's pyogenic granuloma, which had been nonulcerated at index endoscopy, was found to be actively oozing blood from its surface at repeat endoscopy. Surgical excision of gastrointestinal lesions has also been undertaken,5 but with the currently available endoscopic resection techniques, this should be unnecessary for all but large, necrotic, or deep lesions. As these lesions may on occasion involve the full thickness of the luminal wall,5 endoscopic ultrasound is an important tool in determining the depth of extension to determine the suitability of the lesion for endoscopic excision.
In conclusion, we have described for the first time outside of Asia, the clinical presentation of a gastric pyogenic granuloma, its endoscopic and pathologic features, and the utilization of endoscopic ultrasound for the evaluation of endoscopic resectability of these lesions.
References
- 1.Poncet A, Dor L. Botryomycose humaine. Rec Chir. 1897;18:996–1003. [Google Scholar]
- 2.Hartzell MB. Granuloma pyogenicum (botryomycosis of French authors) J Cutan Dis. 1904;22:520–523. [Google Scholar]
- 3.Payson BA, Karpas CM, Exelby P. Intussusception due to pyogenic granuloma of ileum. N Y State J Med. 1967;67:2135–2138. [PubMed] [Google Scholar]
- 4.Imawari H, Fuchigami T, Kobayashi H, Sakai Y. Pyogenic granuloma in the esophagus: report of two cases [in Japanese] Stomach Intest. 1997;32:891–897. [Google Scholar]
- 5.Yao T, Nagai E, Utsunomiya T, Tsuneyoshi M. An intestinal counterpart of pyogenic granuloma of the skin. A newly proposed entity. Am J Surg Pathol. 1995;19:1054–1060. doi: 10.1097/00000478-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Hirakawa K, Aoyagi K, Yao T, Hizawa K, Kido H, Fujishima M. A case of pyogenic granuloma in the duodenum: successful treatment by endoscopic snare polypectomy. Gastrointest Endosc. 1998;47:538–540. doi: 10.1016/s0016-5107(98)70260-3. [DOI] [PubMed] [Google Scholar]
- 7.van Eeden S, Offerhaus GJ, Morsink FH, van Rees BP, Busch OR, van Noesel CJ. Pyogenic granuloma: an unrecognized cause of gastrointestinal bleeding. Virchows Arch. 2004;444:590–593. doi: 10.1007/s00428-004-1013-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen TC, Lien JM, Ng KF, Lin CJ, Ho YP, Chen CM. Multiple pyogenic granulomas in sigmoid colon. Gastrointest Endosc. 1999;49:257–259. doi: 10.1016/s0016-5107(99)70499-2. [DOI] [PubMed] [Google Scholar]
- 9.Lin KJ, Yen TC, Tzen KY. Multiple pyogenic granuloma demonstrated by SPECT using 99Tcm-labelled red blood cells. Br J Radiol. 1999;72:397–399. doi: 10.1259/bjr.72.856.10474504. [DOI] [PubMed] [Google Scholar]
- 10.Okada N, Matsumoto T, Kurahara K, Kanamoto K, Fukuda T, et al. Pyogenic granuloma of the esophagus treated by endoscopic removal. Endoscopy. 2003;35:375. doi: 10.1055/s-2003-38159. [DOI] [PubMed] [Google Scholar]
- 11.Craig RM, Carlson S, Nordbrock HA, Yokoo H. Pyogenic granuloma in Barrett's esophagus mimicking esophageal carcinoma. Gastroenterology. 1995;108:1894–1896. doi: 10.1016/0016-5085(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 12.Manabe T, Goto H, Enya M, Nandate Y, Mizuno S, et al. A case of pyogenic granuloma in the cervical esophagus [in Japanese] Nippon Shokakibyo Gakkai Zasshi. 1998;95:230–232. [PubMed] [Google Scholar]
- 13.Kusakabe A, Kato H, Hayashi K, Igami T, Hasegawa H, et al. Pyogenic granuloma of the stomach successfully treated by endoscopic resection after transarterial embolization of the feeding artery. J Gastroenterol. 2005;40:530–535. doi: 10.1007/s00535-004-1579-3. [DOI] [PubMed] [Google Scholar]
- 14.Viala I, Taillen B, Heuder P, Garnier G, Dumas R, Dujardin P. Une cause inhabituelle d'hemorragie digestive: le botryomycome gastrique. Rev Med Interne. 1993;14:738–739. doi: 10.1016/s0248-8663(05)81242-1. [DOI] [PubMed] [Google Scholar]
- 15.Motohashi Y, Hisamatsu T, Ikezawa T, Matsuoka K, Ogawa S, et al. A case of pyogenic granuloma in the small intestine [in Japanese] Nippon Shokakibyo Gakkai Zasshi. 1999;96:1396–1400. [PubMed] [Google Scholar]
- 16.Carmen Gonzalez-Vela M, Fernando Val-Bernal J, Francisca Garijo M, García-Suárez C. Pyogenic granuloma of the sigmoid colon. Ann Diagn Pathol. 2005;9:106–109. doi: 10.1016/j.anndiagpath.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Hocke M, Bosseckert H. Incorrect macroscopic diagnosis of colonic carcinoma made at endoscopy. Endoscopy. 2004;36:668. doi: 10.1055/s-2004-814534. [DOI] [PubMed] [Google Scholar]
- 18.Hizawa K, Iida M, Matsumoto T, Kohrogi N, Yao T, Fujishima M. Neoplastic transformation arising in Peutz-Jeghers polyposis. Dis Colon Rectum. 1993;36:953–957. doi: 10.1007/BF02050632. [DOI] [PubMed] [Google Scholar]
- 19.Pagliai KA, Cohen BA. Pyogenic granuloma in children. Pediatr Dermatol. 2004;21:10–13. doi: 10.1111/j.0736-8046.2004.21102.x. [DOI] [PubMed] [Google Scholar]
- 20.Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol. 2001;45:1–19. doi: 10.1067/mjd.2001.114595. [DOI] [PubMed] [Google Scholar]
- 21.Meuwissen SG, Willig AP, Hausman R, Starink TM, Mathus-Vliegen EM. Multiple angiomatous proliferetions of ileal stoma following Campylobacter enteritis. Dig Dis Sci. 1986;31:327–332. doi: 10.1007/BF01318126. [DOI] [PubMed] [Google Scholar]
- 22.Cho WY, Ryu CB, Ko BM, Hong SJ, Lee JS, et al. Esophageal pyogenic granuloma. Gastrointest Endosc. 2004;59:539–540. doi: 10.1016/s0016-5107(03)02594-x. [DOI] [PubMed] [Google Scholar]
- 23.Kogawa T, Kogane H, Kayama H. A case of pyogenic granuloma in the stomach [in Japanese] Prog Dig Endosc. 1996;49:112–114. [Google Scholar]
- 24.Okumura T, Tanoue S, Tanaka S. Lobular capillary hemangioma of the esophagus. Acta Pathol Jpn. 1983;33:1303–1308. doi: 10.1111/j.1440-1827.1983.tb02175.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamane T, Omura M, Nakamura M, Sakurai T, Sato Y, et al. A case of esophageal pyogenic granuloma which showed change of form in a short term [in Japanese] Nippon Shokakibyo Gakkai Zasshi. 2003;100:562–566. [PubMed] [Google Scholar]