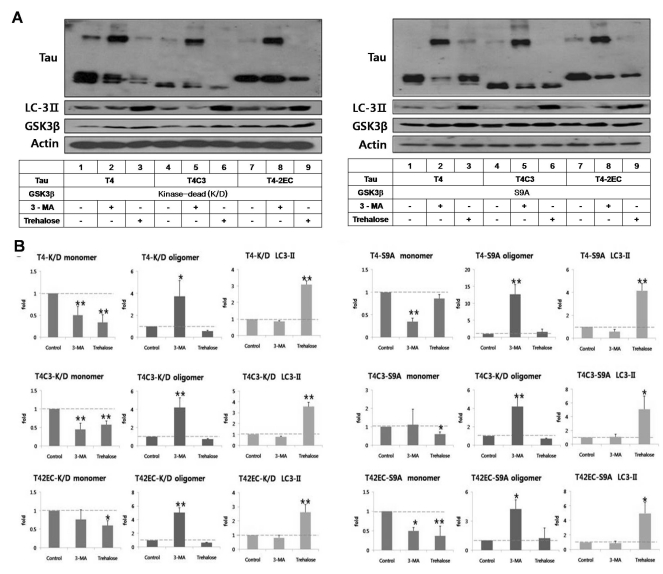
Fig. 3.
GSK3β-phosphorylated T4 is less efficient in trehalose-facilitated autophagic degradation. (A) Representative immunoblots of tau levels with enzyme-inactive GSK3β (kinase-dead (k/D), left) or constitutively active GSK3β (S9A, right), respectively. Tau and each GSK3β constructs were transiently co-transfected into CHO cells. One day after transfection, 3-MA (5 mM) or trehalose (200 mM) was added to the medium and incubated for 24 hrs. Lysates were immunobloted with tau (5A6), LC3, GSK3β, or β-actin antibodies. (B) Quantitative analysis of tau levels. The intensity of monomers and oligomers was quantitated and calculated as a fold to untreated control of each tau. Results are plotted as mean±SD from three independent experiments (n=3). In the presence of kinase-dead GSK3β, 3-MA treatment resulted in the shift of monomeric tau to oligomeric tau in all tau species and trehalose treatment showed significantly decreased intracellular tau level of all tau species. In the presence of active GSK3β, 3-MA treatment resulted in the increased accumulation of oligomeric tau in all tau species. Trehalose treatment resulted in facilitated clearance of T4C3 and T4-2EC. However, phosphorylated T4 by GSK3β was not efficiently degraded, which indicates phosphorylation hinders autophagic degradation. LC3-II levels indicate facilitated autophagic activity with trehalose treatment. Arrows indicate monomeric (~50 kDa) and oligomeric (~150 kDa) tau, respectively. *p<0.05, **p<0.01 to each control.