Figure 4.
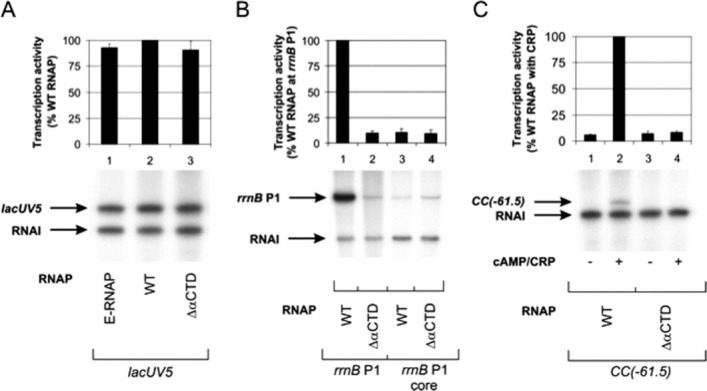
In vitro activity of in vivo-assembled, recombinant E. coli WT and ΔαCTD RNAP preparations. Multiple-round transcription reactions were performed to assess the response of recombinant WT and ΔαCTD σ70-associated holoenzymes to the promoter UP element and the transcription factor CRP. (A) Multiple-round transcription assays were performed at the lacUV5 promoter to determine the concentration of recombinant WT and ΔαCTD RNAPs that gave rise to levels of transcript equivalent to WT endogenous RNAP [E-RNAP (Epicenter); specific activity, 1.4 × 103 U/mg]. (B) Multiple-round transcription assays were performed at the WT rrnB P1 promoter and a derivative of the rrnB P1 promoter lacking an UP element. (C) Multiple-round transcription assays were performed at the synthetic CRP-dependent CC(−61.5) promoter. The different RNAPs are indicated below the gel in each panel. Concentrations of RNAP used are as follows: endogenous WT RNAP, 9.2 nM; in vivo-assembled, recombinant WT RNAP, 9.2 nM; and in vivo-assembled, recombinant ΔαCTD RNAP, 20 nM. Concentrations of supercoiled DNA template used are as follows: lacUV5, 0.2 nM; rrnB P1, 0.6 nM; rrnB P1 core, 0.6 nM; and CC(−61.5), 0.2 nM. The identities of specific transcripts are indicated by arrows [the vector-encoded replication repressor, RNA-I, 108 nucleotides; lacUV5, 131 nucleotides; rrnB P1 promoters, 202 nucleotides; and CC[−61.5], 123 nucleotides]. The abundance of transcripts originating from the lacUV5, rrnB P1, rrnB P1 core, and CC(−61.5) promoters was quantified from three experiments and plotted. In (A), the values were calculated as a percentage of transcript obtained with E-RNAP, whereas in (B) and (C), the values were calculated as a percentage of transcript obtained with WT RNAP and in all cases are presented (with standard deviations) above the appropriate gel, aligned with the corresponding gel lane. Transcription was measured from promoters harbored in the following plasmids: pSR/lacUV5, lacUV5 promoter; pRLG3278, rrnB P1 promoter; pRLG4210, rrnB P1 core promoter; and pSR/CC(−61.5), CC(−61.5) promoter.
