Abstract
Background
With expanding pediatric antiretroviral therapy (ART) access, children will begin to experience treatment failure and require second-line therapy. We evaluated the probability and determinants of virologic failure and switching in children in South Africa.
Methods
Pooled analysis of routine individual data from children who initiated ART in 7 South African treatment programs with 6-monthly viral load and CD4 monitoring produced Kaplan-Meier estimates of probability of virologic failure (two consecutive unsuppressed viral loads with the second being >1,000 copies/ml, after ≥24 weeks of therapy) and switch to second-line. Cox proportional hazards models stratified by program were used to determine predictors of these outcomes.
Results
The 3-year probability of virologic failure among 5485 children was 19.3% (95%CI: 17.6–21.1). Use of nevirapine or ritonavir alone in the initial regimen (compared to efavirenz), and exposure to prevention of mother to child transmission regimens were independently associated with failure (adjusted hazard ratios (95%CI): 1.77(1.11–2.83), 2.39(1.57–3.64) and 1.40(1.02–1.92) respectively). Among 252 children with ≥1 year follow-up after failure, 38% were switched to second-line. Median (IQR) months between failure and switch was 5.7(2.9–11.0).
Conclusion
Triple ART based on nevirapine or ritonavir as a single protease inhibitor appears to be associated with a higher risk of virologic failure. A low proportion of virologically failing children were switched.
Keywords: antiretroviral therapy, virologic failure, children, second-line therapy, resource-limited setting
Introduction
With expanding access to antiretroviral therapy (ART) for HIV-infected children, increasing numbers are likely to experience treatment failure and require second-line regimens. Prior to 2010, WHO pediatric guidelines did not define virologic failure (VF) and viral load monitoring remains unavailable in most resource-limited settings.1–2 In contrast, industrialized country guidelines stipulate strict viral load criteria for switching at thresholds as low as 2 consecutive measurements >400 copies/ml.3–4
Due to poor access to viral load monitoring in resource-limited settings, there is limited published data on VF in children.1 Existing studies are limited by cohort size, exclusive use of non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimens and/or failure definitions based on a single elevated viral load measurement.5–9 Poor access to and lack of experience with second-line therapy, as well as national policies that may restrict second-line use, have resulted in low numbers and proportions of children being switched even in larger cohorts.5–6, 10–14 Predictors of which children are switched in resource-limited settings have therefore not been examined.
The International epidemiologic Databases to Evaluate AIDS (IeDEA) Southern Africa collaboration includes 7 South African pediatric ART programs with data on more than 6000 children who had initiated treatment before 2008.15–16 Regular viral load monitoring (at least 6-monthly) is part of routine ART care in South Africa.17 However, until 2010, national pediatric treatment guidelines did not provide clear direction on management of VF.17 We aimed to examine the probability of VF and its associations, and, in children with VF, to determine the probability of switching to second-line and identify factors that predicted which children were switched.
Methods
Study design, setting and population
Data for this multicenter analysis were collected prospectively at sites. Each site has institutional ethical approval for contribution of data to IeDEA analyses, and transferred data anonymously to the IeDEA data center between May 2007 and February 2008. The analysis included treatment-naïve children (<16 years) initiating ART with ≥3 antiretroviral drugs between June 1999 and February 2008.
Treatment regimens
All treatment sites are part of the South African national treatment program that commenced in April 2004 with the following first-line regimen guidelines: stavudine (d4T), lamivudine (3TC) and either efavirenz (EFV) or, if <3 years/<10kg, a protease inhibitor (PI).17 For most children this was lopinavir/ritonavir (LPV/r), however ritonavir alone (RTV) was recommended for children with tuberculosis or <6 months old.17 The latter two recommendations changed during 2007; LPV/r with additional RTV boosting was introduced for children with tuberculosis and LPV/r dosing recommendations became available for children <6 months old.18–19 Some cohorts introduced these practices before 2007, and also used more varied regimens before commencement of the national program, including NNRTI-based regimens in children <3 years old, RTV alone as the “third drug” in children of all ages, and zidovudine (ZDV) instead of d4T. National guidelines were otherwise adhered to in all provinces and permitted restricted individual drug substitution for intolerance or non-availability of the recommended drug in suitable formulation.17
National guidelines second-line regimens were ZDV + didanosine (ddI) with either LPV/r (EFV-based first-line), or an NNRTI (PI-based first-line).17 The NNRTI was (nevirapine) NVP for children <3 years old at switch, and EFV for older children.17 Decisions to switch could be made by the program clinician without formal Department of Health approval. Second-line regimens were accessible at all sites.
National guidelines advised single dose NVP (sdNVP) for mother and infant for prevention of mother to child transmission (PMTCT), with triple ART for pregnant women with WHO stage 4 disease or CD4 ≤200 cells/μl.20–21 However, in the Western Cape province, PMTCT programs began before national roll-out, with a variety of regimens being used including sdNVP or ZDV from 34 weeks ± sdNVP. Similarly, after national implementation of the sdNVP regimen, the Western Cape province and McCord Hospital used more effective PMTCT regimens (Table 1).22
Table 1.
Characteristics of sites providing ART for children.
| Cohort name and location | Main level of care provided | Type of clinic and payment | Target population | Most likely PMTCT intervention* | First year of paediatric ART provision | Number of children on ART |
|---|---|---|---|---|---|---|
| Harriet Shezi, Chris Hani Baragwanath Hospital, Soweto | All levels | Public and research, Free ART | Children only | sdNVP to mother & infant | 2001 | 1865 |
| Rahima Moosa Mother and Child Hospital, Johannesburg | All levels | Public, Free ART | Children and pregnant women | sdNVP to mother & infant | 1999 | 938 |
| Red Cross Children’s Hospital, Cape Town | Tertiary | Public and research; Free ART | Children only | ZDV from 34 weeks gestation + sdNVP to mother & infant | 2001 | 828 |
| Tygerberg Hospital, Cape Town | Tertiary | Public, Free ART | Adults and children, separate clinics | ZDV from 34 weeks gestation + sdNVP to mother & infant | 2000 | 591 |
| Khayelitsha Community Health Centre, Cape Town | Primary | Public, Free ART | Adults and children, separate clinics | ZDV from 34 weeks gestation + sdNVP to mother & infant | 2001 | 650 |
| Gugulethu Community Health Centre, Cape Town | Primary | Public and research, Free ART | Adults and children, separate clinics | ZDV from 34 weeks gestation + sdNVP to mother & infant | 2001 | 209 |
| McCord Hospital, Durban | Secondary | Government subsidized not for profit hospital, Small co-payment | Adults and children, combined clinics | sdNVP to mother & infant | 2003 | 404 |
| Total | 5485 |
This is the PMTCT intervention available through the public health system within the province in which each site is located. It is not necessarily the intervention that each individual child attending that facility received, as individual program PMTCT regimens varied and mothers may have accessed antenatal care in other provinces/programs.
Key variables
Socio-demographic and clinical data at ART start included age, gender, clinical stage (stage 3 [2002 3-stage WHO classification] and stages 3/4 [2005 4-stage WHO classification] were combined)23–24, exposure to PMTCT regimens and starting regimen. Weight, viral load, CD4 absolute count and percent were available at ART start and 6-monthly thereafter. Access to viral load and CD4 measurement was similar across sites. Viral load measurements were performed using Amplicor 1.5 (Roche Diagnostics) or NucliSens EasyQ assays (bioMerieux), which have good comparability.25 Severe immune suppression was defined according to WHO guidelines.2 “Baseline” measurements were those taken closest to ART initiation and within 6 months (CD4 and viral load) or 2 weeks (weight) prior, to 1 week after commencing ART. Sex-adjusted weight-for-age z-scores (WAZ) were calculated using WHO 2007 reference values for children ≤10 years of age.26
Outcomes
Virologic failure (VF) was defined as 2 consecutive (≤12 months apart) viral load measurements ≥400 copies/ml with the second being >1,000 copies/ml, and both taken after 24 weeks on ART, and not during a treatment interruption. Sensitivity analyses used different thresholds (400, 5,000 and 10,000 copies/ml) to define VF. Children were considered to have switched to second-line if any of the following occurred <1 year after a viral load measurement >400 copies/ml: (i) commencement of ≥2 new drugs including a class-switch from PI to NNRTI or vice versa, (ii) class-switch from NNRTI to PI or vice versa only, with reason documented as treatment failure or (iii) change of both NRTIs and change from RTV to LPV/r with reason documented as treatment failure. Immunologic failure was defined according to South African guidelines criteria for switching as either CD4% below baseline value after 24 weeks of therapy or CD4%<50% of peak value during preceding treatment.
Analysis
Continuous and categorical variables were summarized using medians and interquartile ranges (IQR) and proportions respectively. Kaplan-Meier probabilities of virologic and immunologic failure and switch were estimated. Predictors of failure and switch were determined using Cox-proportional hazards models stratified by site to account for between-site heterogeneity. Only children with ≥6 months of follow-up after failure were included in the switch model. The following variables were included a priori in multivariable models: age, gender and immune suppression at ART initiation (failure model); age, gender and treatment duration at time of failure (switch model). Thereafter multivariable models retained variables with adjusted p-values <0.1. In comparison to known lack of PMTCT exposure, missing PMTCT exposure information had no effect on failure, so these categories were combined. Separate failure models were generated including and excluding WAZ and stage, as missing data for these variables and exclusion of children >10 years old due to lack of WHO WAZ reference values substantially reduced the number of children that could be included in the model. The proportional hazards assumption was met for all models. Statistical analyses were performed using Stata version 10 (STATA Corporation, College Station, TX).
Results
Data from all South African IeDEA sites providing pediatric ART were included (Table 1). This comprised 6266 children of whom 781 (12%) were excluded for the following reasons: missing or inconsistent baseline data (n=85), non-naïve (n=39), mono/dual therapy (n=64) and starting regimen not recorded (n=593). The final dataset comprised 5485 children (49% female) with median (IQR) follow-up of 16 (6–29) months. During follow-up 344 (6%) children died, 411 (7%) were lost to follow-up and 885 (16%) were transferred out after median durations of 1.5, 5.8 and 12.9 months respectively. There were 13877 viral load and 12749 CD4 percent measurements during follow-up with median (IQR) intervals between measurements of 168 (104–190) and 168 (126–197) days respectively.
Most children were severely ill at ART start (Table 2). The median (IQR) age of children commencing ART was 42 (15–82) months. The NRTI backbone was d4T/3TC for 89% of children. The most common “third” drugs were EFV(55%), LPV/r(33%), RTV alone(7%) and NVP(5%).
Table 2.
Characteristics of children at ART initiation, failure (2nd consecutive unsuppressed viral load>1000 copies/ml) and switch.
| Characteristic | All children N=5485 | Children with virologic failure N=523 | Children with virologic failure switched to 2nd line N=145* |
|---|---|---|---|
| Female | 2674 (49%) | 235 (45%) | 51 (35%) |
| Median (IQR) age (months) | |||
| At ART start | 42 (15 – 82) | 37 (13 – 86) | 60 (16 – 94) |
| At failure | NA | 51 (26 – 103) | 74 (29 – 108) |
| < 12 months of age at ART start | 1158 (21%) | 123 (23%) | 26 (18%) |
| Severe immune suppression | |||
| At ART start | 3690/4577 (81%) | 384/447 (86%) | 119/128 (93%) |
| At failure | NA | 184/493 (37%) | 57/138 (41%) |
| Median CD4 percent | |||
| At ART start; n | 12 (7 – 17) | 10 (5 – 15); 408 | 7 (3 – 12); 118 |
| At failure; n | NA | 20 (14 – 26); 464 | 18 (12 – 25); 127 |
| WHO Stage 3 or 4 at ART start | 2887/3832 (75%) | 275/374 (74%) | 88/116 (76%) |
| Viral load > 1 million copies/ml | |||
| At ART start | 777/3745 (21%) | 110/388 (28%) | 29/111 (26%) |
| At failure | NA | 13/524 (2%) | 4/145 (3%) |
| Median (IQR) log viral load | |||
| At ART start; n | 5.3 (4.7 – 5.9) | 5.6 ( 5.0 – 6.1); 388 | 5.6 (5.0 – 6.1); 111 |
| At failure; n | NA | 4.2 (3.7 – 4.9); 524 | 4.4 (3.9 – 5.1); 145 |
| Weight-for-age z-score <-2 | |||
| At ART start | 1759/3646 (48%) | 170/329 (52%) | 46/89 (52%) |
| At failure | NA | 73/385 (19%) | 22/103 (21%) |
| Median (IQR) weight-for-age z-score | −1.93 (−3.36 to −0.96); | −2.08 (−3.43 to −1.07); | −2.14 (−3.31 to −0.96); |
| At ART start; n | 3646 | 329 | 89 |
| At failure; n | NA | −0.87 (−1.74 to −0.12); 385 | −0.79 (−1.74 to −0.13); 103 |
| PMTCT exposure | |||
| Exposed | 556 (10%) | 67 (13%) | 14 (10%) |
| Known unexposed | 1644 (30%) | 176 (34%) | 61 (42%) |
| Exposure status unknown | 3285 (60%) | 280 (53%) | 70 (48%) |
| First-line regimen | |||
| Stavudine & lamivudine based | 4857 (89%) | 420 (80%) | 103 (71%) |
| Nevirapine as third drug | 254 (5%) | 45 (9%) | 20 (14%) |
| Efavirenz as third drug | 3030 (55%) | 251 (48%) | 88 (61%) |
| LPV/r as third drug | 1819 (33%) | 140 (27%) | 10 (7%) |
| Ritonavir alone | 382 (7%) | 88 (17%) | 27 (19%) |
Note: 8 children were switched to second-line who did not meet criteria for virologic failure but had viral load >400 copies/ml preceding switch. These children are excluded from this column.
Virologic failure
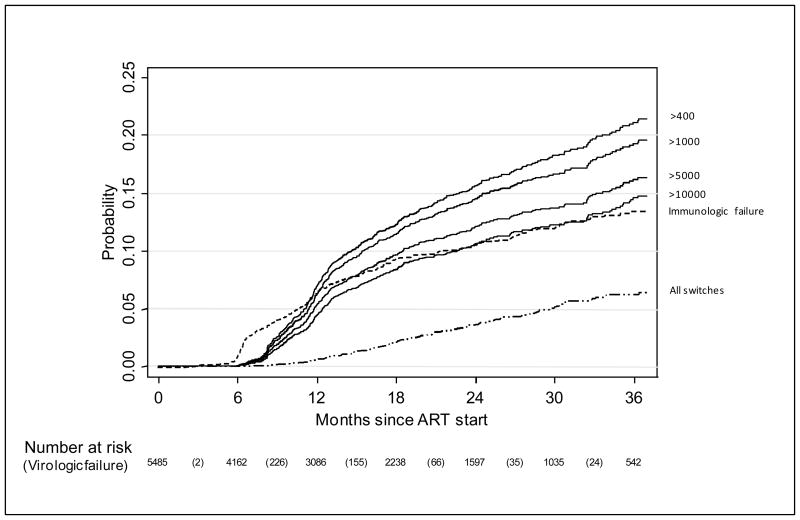
The estimated probability of failure (second elevated value ≥1,000 copies/ml) by 36 months was 19.3% (95%CI: 17.6–21.1, Figure 1). Of the 523 children with VF, 311(59%) had never been virologically suppressed. Among these children whose viral load was never <400 copies/ml, 217 had both baseline and ≥1 subsequent viral load measurement performed between 6 and 15 months on ART, and 121 (55%) showed a virologic response to therapy (≥1 log10 reduction from baseline viral load during the first year on ART). Using different thresholds for the second unsuppressed viral load, the 36-month estimated probability of failure ranged from 14.6% (95%CI: 13.1–16.3) (cut-off=10,000 copies/ml) to 21.1% (95% CI: 19.3–23.0) (cut-off=400 copies/ml) (Figure 1). By one year and 3 years on ART, the estimated probabilities of a single viral load measurement >1,000 copies/ml were 16.9% (95%CI: 15.8–18.1) and 32.1% (95%CI: 30.2–34.1) respectively. By 3 years, 384 children had immunologic failure with an estimated cumulative probability of 12.6% (95% CI: 11.3–13.0). The probability of immunologic failure was lower than that for all definitions of VF, except in the early months as the immunologic failure definition did not require confirmation.
Figure 1.
Kaplan-Meier probability of virologic failure using different viral load values (measured in copies/ml) to define failure, as well as immunologic failure and switch.
Solid lines indicate virologic failure defined as two consecutive unsuppressed viral loads with the second viral load being above the threshold value indicated; dashed line indicates immunologic failure; dash-dot line indicates switch to second line.
Note: The numbers in parentheses in the risk table refer to virologic failure events defined as two consecutive viral load measurements >400 copies/ml with second viral load >1000 copies/ml and this is used as definition of failure in analyses.
In the multivariable model of associations with VF, viral load >1 million copies/ml at ART initiation was the only disease characteristic that predicted failure (Table 3a). After adjustment for gender, age, baseline viral load and immune suppression, failure risk was increased with use of either NVP (adjusted hazard ratio (aHR): 1.77 (95%CI: 1.11–2.83) or RTV alone (aHR: 2.39; 95%CI: 1.57–3.64) compared to EFV in the initial regimen. Known PMTCT exposure was also associated with failure (aHR: 1.40; 95%CI: 1.02–1.92). Results were very similar using different thresholds to define VF. Results were also similar if additionally adjusted for WHO stage and WAZ, neither of which remained independently associated with failure. A further model was developed excluding children with virologic non-response, and results were similar except for an attenuated effect of PMTCT. Results of all additional analyses are shown in an online appendix (eTable 1).
Table 3.
Univariable and multivariable associations with (a) virologic failure in all children commenced on ART and (b) switch to second-line therapy in children with at least 6 months follow-up after a second consecutive unsuppressed viral load, with the second viral load being >1000 copies/ml (Cox proportional hazards models stratified by site).
| (a) | ||||
|---|---|---|---|---|
| Failure definition | 2 consecutive unsuppressed viral loads with the second being >1000 copies/ml | |||
| Characteristic at ART initiation | Unadjusted HR (95% CI) n=5485 | p-value | Adjusted HR (95% CI) n=3605 | p-value |
| Age | ||||
| ≥ 2 years | 1 | <0.001* | 1 | 0.934* |
| 1 – 2 years | 1.37 ( 1.07 – 1.75) | 1.02 ( 0.71 – 1.48) | ||
| < 1 year | 1.83 (1.47 – 2.28) | 1.07 (0.74 – 1.56) | ||
| Female gender | 0.88 ( 0.74 – 1.05) | 0.16 | 0.92 ( 0.75 – 1.13) | 0.442 |
| Viral load > 1 million copies/ml | 2.05 ( 1.63 – 2.58) | <0.001 | 1.67 ( 1.28 – 2.16) | <0.001 |
| Severe immunosupression | 1.48 (1.13 – 1.95) | 0.004 | 1.25 ( 0.94 – 1.68) | 0.131 |
| WHO stage 3 or 4 ( vs 1 or 2)§ | 1.35 (1.06 – 1.73) | 0.016 | ||
| Weight-for-age z-score <-3 § | 1.34 ( 1.06 – 1.69) | 0.014 | ||
| Third drug in regimen | ||||
| Efavirenz | 1 | 1 | ||
| Nevirapine | 1.96 (1.37 – 2.80) | <0.001 | 1.77 (1.11 – 2.83) | 0.016 |
| Lopinavir/ritonavir | 1.36 (1.10 – 1.67) | 0.004 | 1.07 (0.76 – 1.51) | 0.701 |
| Ritonavir alone | 3.06 (2.31 – 4.04) | <0.001 | 2.39 (1.57 – 3.64) | <0.001 |
| PMTCT exposure | ||||
| Unexposed /unknown | 1 | 1 | ||
| Exposed | 1.64 (1.25 – 2.14) | <0.001 | 1.40 (1.02 – 1.92) | 0.039 |
| Year of ART initiation† | ||||
| > 2005 | 1 | |||
| ≤ 2005 | 0.83 (0.66 – 1.04) | 0.1 | ||
| (b) | ||||
|---|---|---|---|---|
| Characteristic | Unadjusted HR (95% CI) n=367 | p-value | Adjusted HR (95%CI) n=229 | p-value |
| Age in years at ART initiation (per 1 year increase in age) | 1.11 (1.05 – 1.16) | <0.001 | 1.03 (0.87 – 1.23) | 0.698 |
| Female gender | 0.64 (0.44 – 0.92) | 0.017 | 0.72 (0.42 – 1.25) | 0.244 |
| Years on treatment at time of failure (per 1 year duration on treatment) | 1.38 ( 1.04 – 1.84) | 0.025 | 1.37 (0.82 – 2.29) | 0.229 |
| Log10 viral load at failure (per 1 log increase) | 1.43 ( 1.15 – 1.78) | 0.002 | 1.55 ( 1.11 – 2.16) | 0.01 |
| Current§ immunological failure* | 1.08 ( 0.67 – 1.75) | 0.75 | ||
| Current§ CD4 % <25 | 2.45 ( 1.56 – 3.84) | <0.001 | 1.94 (1.07 – 3.52) | 0.029 |
| Current CD4% decline >1unit /month† | 4.37 ( 2.14 – 8.92) | <0.001 | 6.44 (2.15 – 19.25) | 0.001 |
| Current§ weight-for-age z-score (per 1 unit increase in z-score) | 0.94 (0.78 – 1.13) | 0.521 | 1.14 ( 0.90 – 1.43) | 0.281 |
| Current weight-for-age z-score decline > 0.1 units/month† | 1.62 (0.64 – 4.11) | 0.313 | 2.10 (0.58 – 7.58) | 0.257 |
| Viral load decline < 1 log10 since ART start* | 0.88 (0.55 – 1.41) | 0.596 | ||
| PI-containing regimen | 0.39 ( 0.25 – 0.60) | <0.001 | 0.40 (0.17 – 0.91) | 0.03 |
| Year of ART initiation* | ||||
| > 2005 | 1 | |||
| ≤ 2005 | 1.10 (0.52 – 2.32) | 0.803 | ||
p-values derived from Wald’s test
Not included in multivariable model as missing information would have limited overall number of children that could be included
Not included in multivariable model as p>0.1 after adjustment for other variables in the model
Not included in multivariable model
Measurement taken at time of switch
Difference between measurements taken at time of switch and preceding visit
Note: Reasons for children having less than 6 months follow-up after failure (n=156), were death (n=5; 3%), loss to follow-up (n=15; 10%), transfer out (n=13; 8%) and failure occurring less than 6 months before database closure (n=123; 79%).
Switching to second-line
The estimated probability of switching to second-line by 3 years after ART initiation for all children was 6.2% (95% CI: 5.2–7.5, Figure 1). Of the 153 children switched, 8 did not meet the VF criteria because there was only one unsuppressed viral load measurement (n=7) or consecutive measurements were both before 24 weeks on ART (n=1). Of 252 children with ≥1 year of follow-up after failure, 38% (95%CI: 32%–45%) were switched. The median (IQR) time to switch from failure was 5.7 (2.9–11.0) months and from first unsuppressed viral load was 9.5 (5.5–14.6) months. The median (IQR) interval between consecutive unsuppressed viral load measurements was 3.2 (2.5–5.4) months.
Most second-line regimens included ddI as one of the NRTIs (108/153; 71%). Other NRTIs included ZDV(66%), 3TC(25%); d4T(21%); abacavir(13%), and tenofovir(1%). The “third drug” in the regimen was LPV/r for 74% of children.
After adjustment for age at ART initiation, gender and treatment duration, children with more severe or progressive disease from the time of failure (higher viral load, CD4% <25 at switch, CD4% decline >1 percentage point per month between switch date and preceding visit) were more likely to be switched, while taking a PI-based initial regimen was negatively associated with switch (aHR 0.40; 95%CI: 0.17–0.91) (Table 3b). Failure to initially attain viral load <400 copies/ml after starting ART was not associated with switch in univariable or multivariable analysis. (aHR: 1.02; 95%CI: 0.52–1.99).
Discussion
Main findings
This study reports in detail on confirmed VF and switching in children on ART in a large African multicenter study where routine viral load monitoring was available. One in five children had met the analysis definition of confirmed VF by three years on ART. Baseline viral load >1million copies/ml, use of either NVP, or RTV as a sole PI, as well as PMTCT exposure independently predicted failure. Less than half of children with ≥1 year of follow-up after failure were switched, with a median interval between failure and switch of 5.7 months. Across all sites, current poor immunologic and virologic status together with being on an NNRTI-based regimen favored switch.
Time to virologic failure
Previous studies from Thailand and Uganda with all children on NNRTI-based regimens and failure defined using a single viral load measurement, reported similar proportions of children with VF at 12 months on ART as we report at 36 months using confirmed measurements.5–6 Similarly, a recent cohort study found the frequency of consecutive viral load measurements >400 copies/ml among 116 children with follow-up ≥6 months to be 17%.8 Nevertheless, the cumulative probability of a single elevated viral load measurement after one year on ART in our study (16%) is similar to the Thai and Ugandan studies. In contrast, prevalence of a single viral load measurement >400 copies/ml was 32% at a Tanzanian pediatric clinic, however 12% of those with VF were on second-line.9 Notwithstanding, our study differs from these with use of PI-based first-line therapy, and possible differences in PMTCT exposure and adherence.
For those on NNRTI-based regimens, the confirmation of VF following adherence optimization, as reported in this study, is likely to identify patients who are truly failing with resistance to ≥1 drug in the regimen. For example, an adult study from South Africa showed that 86% of patients with confirmed viral load >1000 copies/ml had therapy-limiting NNRTI mutations.27 Among children with VF in the Thai study, 89% and 97% had major NRTI and NNRTI resistance mutations respectively. We had no access to resistance testing for children failing therapy, and the prevalence of resistance among children on PI-based regimens with confirmed VF in our context remains unknown.
Comparisons with rich countries are difficult due to differences in age at ART commencement, previous mono- or dual-therapy, follow-up duration and first-line regimens. The UK Collaborative HIV Paediatric Study (CHIPS) reported 32% of 595 children having VF after a median follow-up of 3 years, while a Dutch cohort of 39 children on nelfinavir-based ART reported 74% virologic failure-free survival after 48 weeks.28–29
First-line regimen choice
The association between NVP-containing regimens and VF concurs with findings from previous pediatric and adult studies.5–6, 30–31 It has been suggested that NVP may be under-dosed in children taking split adult fixed dose combination tablets, however, in South Africa, NVP is administered to children as a single drug in syrup/tablet form.5–6 Children may harbor resistance from unrecorded exposure to sdNVP, and subsequent NVP-based ART would be expected to result in poor virologic outcomes.32–33 Although the majority of sdNVP-exposed children in this cohort would have commenced PI-based regimens, it is likely that some initiated NNRTI-based regimens due to site variation in regimen use before national guidelines recommendations. This is supported by both the finding of an association between PMTCT exposure and subsequent failure, despite PMTCT under-ascertainment, as well as attenuation of this effect when those without virologic response to ART were excluded. This attenuation is expected as children with NVP resistance would most likely be virologic non-responders.
Despite our inability to adjust for potential confounding by concomitant tuberculosis and other confounding by indication, our findings suggest that RTV as the sole PI is indeed associated with failure. RTV is unpleasant tasting, associated with poor adherence, and results in a greater accumulation of major PI resistance mutations in comparison with LPV/r.34, 35 However, as RTV use would have been more common in children with tuberculosis, we cannot exclude that worse outcomes may have been due to tuberculosis itself or the increased medication burden of ART combined with anti-tuberculous therapy.
Switching to second-line
This study reflects clinical practice in a setting with viral load monitoring but no supporting national or WHO guidelines regarding management of children with VF. This is reflected in the low proportion children switched after failure, and the delay between VF and switch. Heterogeneity in switching practice was also seen in the CHIPS study with nearly half of children with VF being switched before the date of first viral load >1000 copies/ml, but an equal proportion on first-line ≥6 months thereafter.28 In our study, service factors may contribute to the delay; clinical appointments are often 3- monthly with results only available for decision-making at subsequent appointments.
Nevertheless, less than half of children with confirmed VF for ≥1 year were switched, and those who were switched were on a failing regimen for a median of 10 months after the first elevated viral load measurement. In this study, factors other than VF were associated with being switched, including initial regimen, disease severity and progressive immunological decline.
Reluctance to switch a young child failing therapy without thorough assessment of adherence is reasonable in the context of access only to unpleasant second-line regimens, with no third-line/salvage therapy. In this respect, reduced switching of children on PI-based regimens is consistent with knowledge that viral escape is more likely due to poor adherence than resistance.34 Nevertheless, poor access to a wider range of second-line drugs, particularly for children failing first-line PI-based regimens following sdNVP exposure, may result in an understandable reluctance to switch children to a drug to which their virus may be resistant.
If the intention of treatment guidelines is to avoid prolonged viraemia, this study suggests the need for more intensive monitoring and adherence interventions soon after a single elevated viral load. The PENPACT1 trial recently reported similar outcomes overall for children switched at viral load measurements of 1,000 or 30,000 copies/ml, however highlighted the importance of adherence interventions after initial elevated viral load measures.36–37 In addition, children on NNRTI-based therapy switched at 30,000 copies/ml accumulated more NRTI-resistance mutations compared to those switched at 1,000 copies/ml, suggesting that switching guidelines should be tailored according to regimen.36–37 In large programs, viral load monitoring could additionally be used to manage patient load by stratifying risk. More clinical and adherence input could be given to unsuppressed patients while those with sustained virologic suppression could be managed less intensively.7, 38
Strengths and limitations
This is a large combined cohort of children across many sites providing different levels of care. In addition, viral load measurements were available for >75% of children in care at each 6-monthly duration, and it was possible to use as an outcome confirmed virologic failure rather than a single measure. The large number of infants and inclusion of a PI in first-line enabled us to examine the effect on virologic outcome of RTV as the sole PI, as well as LPV/r in comparison to NVP or EFV as components of first-line regimens. The size of the cohort resulted in a relatively large absolute number of failures and switches, permitting investigation of switching practice.
Despite the study size, and the general application of the public health approach to ART provision, the study cohorts, being relatively well-resourced and urban, may not be representative of all sites across the region or even South Africa.
Data was collected in the context of routine care in busy clinics. There is limited data on key possible predictors of VF such as tuberculosis co-infection, adherence and PMTCT, as well as on clinical events. Missing data on other variables limited the range of variables and number of children that could be included in multivariable models. Tuberculosis co-infection not only affects first-line regimen choice, but may impact on virologic outcomes directly or through drug-drug interactions or reduced adherence. We could not explore the extent to which our observed associations with failure were mediated through poor adherence, due to limited data. PMTCT exposure data was only recorded for 40% of children. Furthermore, exact PMTCT regimens were not recorded, so the effect of different regimens could not be examined. The effect of severe clinical disease at ART initiation on VF may have been reduced by combining stage 3 and 4 disease. Due to lack of detailed clinical event data as well as confounding by indication (with sicker children being preferentially switched), we were unable to determine the clinical consequences of delayed switching.
Conclusion
This study demonstrates the probability of VF in children on ART in South Africa at 3 years to be nearly 20%. The time between failure and switch and low proportion of children switched to second-line in this and other studies supports use of clearer definitions of VF and clinical practice guidelines for managing children with unsuppressed viral load, tailored to starting regimen. In addition, access to second-line drugs for PMTCT exposed children failing PI-based ART is important for better pediatric HIV care in the countries where the majority of HIV-infected children reside.
Supplementary Material
Acknowledgments
Sources of support: This study was supported by the National Institute of Allergy and Infectious Diseases and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant 1 U01 AI069924-01).
This study was supported by the National Institute of Allergy and Infectious Diseases and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant 1 U01 AI069924-01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank all the children whose data was used in this analysis, as well as their caregivers. We also thank all staff at participating sites for preparation of data contributed to the IeDEA Southern Africa collaboration. Many thanks to Nicola Maxwell for preparing the combined data for analysis, to Morna Cornell and Claire Graber for project management and to Francesca Little for advice on the analysis. Mary-Ann Davies had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors state that they have no conflict of interest.
IeDEA Southern Africa Steering Group
Member Sites: Anna Coutsoudis, PMTCT Plus, Durban, South Africa; Diana Dickinson, Gaborone Independent Hospital, Gaborone, Botswana; Brian Eley, Red Cross Children’s Hospital, Cape Town, South Africa; Lara Fairall, Free State provincial ARV roll-out, South Africa; Tendani Gaolathe, Princess Marina Hospital, Gaborone, Botswana; Janet Giddy, McCord Hospital, Durban, South Africa; Timothy Meade, CorpMed Clinic, Lusaka, Zambia; Patrick MacPhail, Themba Lethu Clinic, Helen Joseph Hospital, Johannesburg, South Africa; Lerato Mohapi, Perinatal HIV Research Unit, Johannesburg, South Africa; Margaret Pascoe, Newlands Clinic, Harare, Zimbabwe; Hans Prozesky, Tygerberg Academic Hospital, Stellenbosch, South Africa; Harry Moultrie, Enhancing Children’s HIV Outcomes (Harriet Shezi Children’s Clinic, Chris Hani Baragwanath Hospital, Soweto); Karl Technau, University of Witwatersrand Paediatric HIV Clinics (Empilweni Clinic, Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa; Gilles van Cutsem, Khayelitsha ART Programme and Médecins sans Frontières, Cape Town, South Africa; Paula Vaz, Paediatric Day Hospital, Maputo, Mozambique; Ralf Weigel, Lighthouse Clinic, Lilongwe, Malawi; Robin Wood, Gugulethu and Masiphumelele ART Programmes, Cape Town, South Africa.
Central Team: Martin Brinkhof, Matthias Egger, Beatrice Fatzer, Claire Graber and Olivia Keiser, Institute of Social and Preventive Medicine, University of Bern, Bern, Switzerland; Andrew Boulle, Morna Cornell, Mary-Ann Davies, Nicola Maxwell, Landon Myer and Anna Grimsrud, School of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa.
Footnotes
Previous presentations: This study was presented at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention in Cape Town, 2009 (abstract number 1759) and at the 1st International Workshop on HIV Pediatrics in Cape Town 2009 (abstract number O-01)
References
- 1.The KIDS-ART-LINC Collaboration. Low risk of Death, but Substantial Program Attrition, in Pediatric Treatment Cohorts in Sub-Saharan Africa. J Aquir Immune Defic Syndr. 2008;15(5):523–531. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 2.WHO. [Accessed 2007/09/02.];Antiretroviral therapy of HIV infection in infants and children: towards universal access. 2006 http://www.who.int/hiv/pub/paediatric/infants/en/index.html. [PubMed]
- 3.Paediatric European Network for Treatment of Aids. [Accessed 2009/11/01];PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. 2008 http://www.pentatrials.org/guide09.pdf.
- 4.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. [Accessed 2008/12/17.];Guidelines for the use of antiretroviral agents in Pediatric HIV infection. 2008 http://AIDSinfo.nih.gov.
- 5.Jittamala P, Puthanakit T, Chaiinseeard S, et al. Predictors of virologic failure and genotypic resistance mutation patterns in Thai children receiving non0nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Pediatr Infect Dis J. 2009;28(9):826–830. doi: 10.1097/INF.0b013e3181a458f9. [DOI] [PubMed] [Google Scholar]
- 6.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of long-term viral failure among ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46(2):187–193. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 7.Germanaud D, Derache A, Traore M, et al. Level of viral load and antiretroviral resistance after 6 months of non-nucleoside reverse transcriptase inhibitor first-line treatment in HIV-1-infected children in Mali. J Antimicrob Chemother Jan. 2010;65(1):118–124. doi: 10.1093/jac/dkp412. [DOI] [PubMed] [Google Scholar]
- 8.Ruel TD, Achan J, Charlebois E, et al. Sustained viremia is common among HIV-infected Ugandan children receiving antiretroviral therapy and not detected by WHO CD4 criteria. Paper presented at: CROI; San Francisco, USA. 2010. [Google Scholar]
- 9.Emmett SD, Cunningham C, Mmbaga BT, et al. Predicting virologic failure among HIV-1 infected children receiving antiretroviral therapy in Tanzania: a cross-sectional study. J Acquir Immune Defic Syndr. 2010;54(4):368–375. doi: 10.1097/QAI.0b013e3181cf4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk MB, Linde R, Wintergerst U, et al. Preliminary experiences with triple therapy including nelfinavir and two reverse transcriptase inhibitors in previously untreated HIV-infected children. AIDS. 1999 Sep 10;13(13):1653–1658. doi: 10.1097/00002030-199909100-00008. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F, Haberer JE, Zhao Y, et al. Chinese pediatric highly active antiretroviral therapy observational cohort: a 1-year analysis of clinical, immunologic, and virologic outcomes. J Acquir Immune Defic Syndr. 2007 Dec 15;46(5):594–598. doi: 10.1097/QAI.0b013e318158c08e. [DOI] [PubMed] [Google Scholar]
- 12.Sutcliffe CG, van Dijk JH, Bolton C, et al. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis Aug. 2008;8(8):477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 13.Janssens B, Raleigh B, Soeung S, et al. Effectiveness of highly active antiretroviral therapy in HIV-positive children: evaluation at 12 months in a routine program in Cambodia. Pediatrics. 2007;120:e1134–e1140. doi: 10.1542/peds.2006-3503. [DOI] [PubMed] [Google Scholar]
- 14.Bock P, Boulle A, White C, et al. Provision of antiretroviral therapy to children within the public sector of South Africa. Trans R SocTrop Med Hyg. 2008;102(9):905–911. doi: 10.1016/j.trstmh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Davies M, Keiser O, Technau K, et al. Outcomes of the South African National Antiretroviral Treatment (ART) programme for children - The IeDEA Southern Africa Collaboration. S Afr Med J. 2009;99(10):730–737. [PMC free article] [PubMed] [Google Scholar]
- 16.Fenner L, Brinkhof M, Keiser O, et al. Early mortality and loss to follow-up in HIV-infected children starting antiretroviral therapy in Southern Africa. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3181e0c4cf. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Department of Health South Africa. Guidelines for the management of HIV-infected children in South Africa. Vol. 1. Jacana: 2005. [Google Scholar]
- 18.Ren Y, Nuttall J, Egbers C, et al. Effet of rifampicin on lopinavir pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr. 2008;47:566–269. doi: 10.1097/QAI.0b013e3181642257. [DOI] [PubMed] [Google Scholar]
- 19.Chadwick E, Capparelli EV, Yogev R, et al. Pharmacokinetics, safety and efficacy of lopinavir/ritonavir in infants less than 6 months of age: 24 week results. AIDS. 2008;22:249–255. doi: 10.1097/QAD.0b013e3282f2be1d. [DOI] [PubMed] [Google Scholar]
- 20.Jackson DJ, Chopra M, Doherty TM, et al. Operational effectiveness and 36 week HIV-free survival in the South African programme to prevent mother-to-child transmission of HIV-1. AIDS. 2007;19(4):509–516. doi: 10.1097/QAD.0b013e32801424d2. [DOI] [PubMed] [Google Scholar]
- 21.National Department of Health South Africa. National Antiretroviral Treatment Guidelines. Jacana: 2004. [Google Scholar]
- 22.Coetzee D, Hilderbrand K, Boulle A, et al. Effectiveness of the first district-wide programme for the prevention of mother-to-child transmission of HIV in South Africa. Bull World Health Organ. 2005;83(7):489–494. [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Scaling up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for a Public Health Approach, 2003 Revision. Geneva, Switzerland: 2004. [Google Scholar]
- 24.WHO. [Accessed 2007/08/05.];Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance. 2005 www.who.int/hiv/pub/guidelines/clinicalstaging.pdf.
- 25.Stevens W, Wiggill T, Horsfield P, et al. Evaluation of the NucliSensEasyQ assay in HIV-1-infected individuals in South Africa. Journal of Virological Methods. 2005;124:105–110. doi: 10.1016/j.jviromet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 26.WHO. [Accessed 2008/11/23.];The WHO Child Growth Standards. 2007 http://www.who.int/childgrowth/en/
- 27.Orrell C, Walensky R, Losina E, et al. HIV type-1 clade C resistance genotypes in treatment-naive patients and after first virological failure in a large community antiretroviral therapy programme. Antivir Ther. 2009;14(4):523–531. [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KJ, Lyall H, Walker AS, et al. Wide disparity in switch to second-line therapy in HIV infected children in CHIPS. Paper presented at: Eighth International Congress on Drug Therapy in HIV infection; 11/12/2006; Glasgow, UK. [Google Scholar]
- 29.Scherpbier HJ, Bekker V, Van Leth F, et al. Long-term experience with combination antiretroviral therapy that contains nelfinavir for up to 7 years in a pediatric cohort. Pediatrics. 2006;117:e528–e536. doi: 10.1542/peds.2005-1272. [DOI] [PubMed] [Google Scholar]
- 30.Nachega JB, Hislop M, Dowdy DW, et al. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146(8):564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 31.Boulle A, Van Cutsem G, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300(5):530–539. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 32.Arrive E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36(5):1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 33.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010 Oct 14;363(16):1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Zyl GU, Van der Merwe L, Cotton M, et al. Protease-inhibitor resistance in South African children exposed to ritonavir as single protease inhibitor compared to a lopinavir/ritonavir regimen. Paper presented at: IAS; 2009; Cape Town, South Africa. [Google Scholar]
- 35.Davies M, Boulle A, Fakir T, et al. Adherence to antiretroviral therapy in young children in Cape Town, South Africa. BMC Pediatrics. 2008:8. doi: 10.1186/1471-2431-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.PENPACT1. A phase II/III randomised, open-label trial of combination antiretroviral regimens and treatment-switching strategies in HIV-1-infected antiretroviral naïve children. Paper presented at: 2nd International Workshop on HIV Pediatrics; 2010; Vienna, Austria.. [Google Scholar]
- 37.PENPACT1. A phase II/III randomised, open-label trial of combination antiretroviral regimens and treatment-switching strategies in HIV-1-infected antiretroviral naïve children. Paper presented at: IAS; 2010; Vienna, Austria. [Google Scholar]
- 38.Ford N, Calmy A. Improving first-line antiretroviral therapy in resource-limited settings. Curr Opin HIV AIDS. 2010;5(1):38 – 47. doi: 10.1097/COH.0b013e3283339b41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.