Abstract
It is common knowledge that many of the cell components we study today were discovered more than a century ago. Some have been renamed due to a newer understanding of their physiology or composition, and in some cases the old terminology is abandoned. However, it is unusual to find a structure that has not been renamed but simply forgotten. This appears to be the case for the nucleolinus, discovered at least 150 years ago and studied by Agassiz, Haekel, Montgomery and others until it virtually dropped from the literature in the early 1970s. The nucleolinus was thought to have a role in cell division, but with little knowledge of its composition and no molecular markers (until recently) available for its study, we do not know if the nucleolinus is a ubiquitous structure or an antiquated descriptor. This brief article relates most of what we know about the nucleolinus and where to find more information. Our growing knowledge concerning the role of the closely allied nucleolus in cell cycle regulation suggests that renewed study of the nucleolinus will yield important information about the biogenesis and evolution of the cell division apparatus.
Key words: centrosome, spindle, centrosomal RNA, cell division, nucleolus
Definition and History
At the time most readers first raise this article to eye level, they will not have heard of the nucleolinus. It is not, however, a recent contrivance or a new twist in an old story. Nor, as far as we can tell, does it represent the resurrection of long-abandoned terminology for an otherwise commonly known structure. Remarkably, the nucleolinus is a bona fide cellular compartment with a history going back at least 150 years1 that has been virtually forgotten. We anticipate that interest in this structure will be reinvigorated given recent findings that it2 and the nucleolus itself3–5 are directly involved in cell cycle regulation. However for the present, nothing is better suited to relay everything we know about the nucleolinus than a mini-review.
Structures under the name of nucleolinus have been reported in a wide variety of cells. This includes model systems with which we are all familiar, from marine invertebrate eggs (Montgomery, 1898,6 and references therein) to vertebrate somatic7 and mammalian tumor cells.8 Because we know so little about its composition and have no molecular markers for the nucleolinus (until now), we cannot even be sure that past investigators were describing analogous structures in their own experimental systems. The nucleolinus is closely apposed to the nucleolus and often cannot be distinguished without certain histochemical stains. Histochemical stains in general, let alone these particular preparations, are no longer in common use for basic cell biology research. As a result, many investigators only barely familiar with the structure consider it to be part of the nucleolus. This may in fact be the case. Yet, it is differentiated from the nucleolus in species as divergent as frogs and humans histochemically, behaviorally and morphologically, suggesting there may be more to this story that we have overlooked.
The most comprehensive analysis of the nucleolinus appears in a series of reports by Love and colleagues. Love and Liles9 developed a method for the differentiation of nucleoprotein complexes with Toluidine Blue and ammonium molybdate and, using a variety of normal and transformed mammalian cells, stained the nucleolinus metachromatically against the uniformly green background of the nucleolus. The authors observed this metachromatic compartment to increase during prophase and then dissipate as the cell progressed through anaphase and telophase. This correlates with the behavior of the nucleolinus in surf clam (Spisula) oocytes,10 which are arrested in prophase I of meiosis and display a prominent nucleolinus (Fig. 1). After the oocyte is fertilized or parthenogenetically activated, the nucleolus dissipates quickly, within approximately 6 minutes. The nucleolinus continues as a distinct morphological entity for approximately 5 more minutes, and then also disappears. The nucleolus and nucleolinus are therefore demonstrably separated under normal physiological conditions in these cells. A physiological separation between the nucleolus and nucleolinus was also reported by Love and Wildy11 in Herpes virus-infected HeLa cells. The authors describe enlargement of nucleolini and their extrusion from the nucleolus as the first visible abnormality following viral infection.
Figure 1.

Unactivated surf clam (Spisula solidissima) oocyte. A large tetraploid nucleus (germinal vesicle; GV) is present, within which lie a prominent nucleolus (arrow) and nucleolinus (arrowhead). The nuclear envelope begins to disintegrate within the first two minutes of activation and the GV is indistinguishable by 10–12 minutes post-fertilization. The nucleolus disappears within 5–6 minutes of fertilization, and the nucleolinus persists for 4–5 minutes by itself. Size bar = 15 µm.
Physiological Significance
The little information we have on the possible functions of the nucleolinus is mostly descriptive. From his observations on invertebrate eggs, Lavdowsky thought that it was a precursor to the centrosomes. Allen10,13 stated that the nucleolinus in Spisula oocytes remains in the vicinity of the forming maturation spindle and that “observations on fixed material indicate a spindle forming role for the nucleolinus.” However, this was a written description with no supporting data presented. Love and Wildy11 observed changes in nucleolini corresponding with perturbations in cell division. As did Allen,10,13 the authors speculated on a functional relationship, but the hypothesis is supported only by correlative data. Love14 reported that changes in nucleolinar morphology occur in aneuploid and virally-transformed cells, and Mironescue et al. observed similar results in rat liver cells after administration of liver carcinogens.
Using time lapse microscopy on living, fertilized Spisula oocytes, Allen's observations10,13 that the nucleolinus remains in the vicinity of the spindle pole were recently confirmed.2 Indeed, the position of the nucleolinus could be used to predict the position of the later-appearing meiotic spindle poles and the point on the oocyte surface from which the polar bodies would be ejected. The site of polar body ejection, coincidentally, foretells the major embryonic axes of molluscan embryos. Moreover, Alliegro and Alliegro16 reported that specific RNAs later localized with the centrosome (cnRNAs) are present in the nucleolinus of Spisula oocytes before centrosomes are formed, and the centrosomes and meiotic spindle come to lie in a matrix of nucleolinar RNA following fertilization.2 Finally, laser microsurgery on the nucleolinus was shown to result in cell division defects, providing the first experimental evidence of a role for this structure in cell division.
Composition of the Nucleolinus
Love and Walsh showed that the nucleolinus contains RNA and protein. These authors could not detect DNA in the structure. Specific molecules associated with the nucleolinus were not identified until very recently, so other than histochemical differentiation there has been no way to distinguish the content of the nucleolinus from the nucleolus or other cellular structures. However, nucleolinar-specific probes are now becoming available and it is clear the structure contains RNAs not present in the nucleolus or elsewhere in the cell (Fig. 2A–C). In addition, certain protein components of the nucleolus are excluded from the nucleolinus (Fig. 2D–F), and others present in the nucleolinus are excluded from the nucleolus (Fig. 2G–L). Thus, the nucleolinus and nucleolus may be integrated morphologically in many cell types, but there are clear examples of specific molecules to distinguish these two compartments in at least some species.
Figure 2.
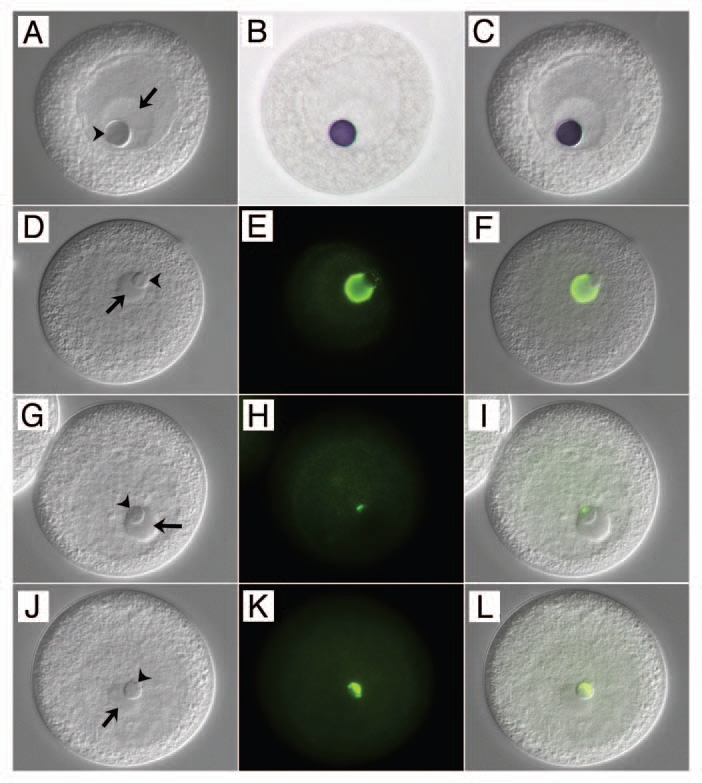
Distinguishing the nucleolinus from the nucleolus at the molecular level. The left-hand column of panels (A, D, G and J) are DIC images of oocytes. The middle column (B, E, H and K) shows the various probe signals in color brightfield (B) or immunofluorescence (E, H and K). The right hand column of panels (C, F, I and L) are overlays showing the specific labeling patterns against the DIC backgrounds. (A–C) in situ hybridization showing the localization of NLi-1 RNA to the nucleolinus (arrowhead) in an unactivated oocyte, and its absence from the nucleolus (arrow). Note that the nucleolus and nucleolinus swell slightly during the three-day in situ hybridization regimen. (D–F) localization of an antigen recognized by mcAb NLi-26 present in the nucleolus, but absent from the nucleolinus. (G–I) localization of an antigen recognized by mcAb NLi-9, which is absent from the nucleolus but localizes to a small zone near one pole of the nucleolinus in unactivated oocytes. After fertilization, the NLi-9 antigen expands across the nucleolinus (J–L). Oocytes shown in (D–L) are slightly compressed under the cover glass; the borders of the GV are therefore not as clearly distinguishable.
In the Spotlight or Back to the Museum?
None of the recent findings on the nucleolinus are revelations. As happens so frequently, our 19th century and early 20th century predecessors, armed with simple compound microscopes and camera lucida, were on the right track. Although the mechanism appears to be more complicated than a simple progenitor-progeny relationship, Lavdowsky12 may have been correct that the nucleolinus is a centrosomal precursor. Observations on the behavior and content of the nucleolinus certainly support the hypothesis that it is involved in cell division. If the nucleolinus is best considered to be part of the nucleolus, then perhaps it is that part directly involved in cell cycle regulation, distinct from nucleolar function in ribosome biogenesis. However, we cannot rule out that the nucleolinus should be considered a separate compartment or that it has origins as a distinct, primitive organelle. We can begin to answer these questions of function and evolutionary history only when we know more about the occurance and molecular composition of the nucleolinus in diverse taxa.
Acknowledgements
Work from the Alliegro laboratory on the nucleolinus and centrosomal RNAs described in this paper was supported by grants from the NIH (GM088503) and NSF (MCB0843092) to Mark Alliegro. The author thanks Mary Anne Alliegro for reading and commenting on the manuscript.
Abbreviations
- cnRNA
centrosome-associated RNA
- NLi
nucleolinar
References
- 1.Agassiz L. Part IIL Embryology of the turtle. Contributions to the Natural History of the United States of America. First Monograph. Boston: Little Brown and Company; 1857. [Google Scholar]
- 2.Alliegro MA, Henry J, Alliegro MC. Rediscovery of the nucleolinus, a dynamic RNA-rich organelle associated with the nucleolus, spindle and centrosomes. Proc Natl Acad Sci USA. 2010;107:13718–13723. doi: 10.1073/pnas.1008469107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaulden ME, Perry RP. Influence of the nucleolus on mitosis as revealed by ultraviolet microbeam irradiation. Proc Natl Acad Sci USA. 1958;44:553–559. doi: 10.1073/pnas.44.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ugrinova I, Monier K, Ivaldi C, Thiry M, Storck S, Mongelard F, et al. Inactivation of nucleolin leads to nucleolar disruption, cell cycle arrest and defects in centrosome duplication. BMC Mol Biol. 2007;8:66. doi: 10.1186/1471-2199-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pederson T, Tsai YL. In search of non-ribosomal nucleolar protein function and regulation. J Cell Biol. 2009;184:771–776. doi: 10.1083/jcb.200812014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montgomery TH. Comparative cytological studies, with especial regard to the morphology of the nucleolus. J Morph. 1898;15:265–582. [Google Scholar]
- 7.Carleton HM. Observations on an intra-nucleolar body in columnar epithelium cells of the intestine. Q J Microsc Sci. 1920;2-64:329–341. [Google Scholar]
- 8.Love R, Soriano RZ. Correlation of nucleolini with fine structural constituents of cultured normal and neoplastic cells. Cancer Res. 1971;31:1030–1037. [PubMed] [Google Scholar]
- 9.Love R, Liles RH. Differentiation of nucleoproteins by inactivation of protein-bound amino groups and staining with toluidine blue and ammonium molybdate. J Histochem Cytochem. 1959;7:164–181. doi: 10.1177/7.3.164. [DOI] [PubMed] [Google Scholar]
- 10.Allen RD. Fertilization and artificial activation in the egg of the surf calm, Spisula solidissima. Biol Bull. 1953;105:213–239. [Google Scholar]
- 11.Love R, Wildy P. Cytochemical studies of the nucleoproteins of HeLa cells infected with herpes virus. J Cell Biol. 1963;17:237–254. doi: 10.1083/jcb.17.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavdowsky M. Formation of the chromatic and achromatic substances in animal and plant cells. Merkel Bonnet's Anat Hefte. 1894;4:13. [Google Scholar]
- 13.Allen RD. The role of the nucleolus in spindle formation. Biol Bull. 1951;101:214. [Google Scholar]
- 14.Love R. Anisonucleolinosis in mammalian cell cultures. Natl Canc Inst Monographs. 1966;23:167–180. [PubMed] [Google Scholar]
- 15.Mironescue S, Encut I, Mironescu K, Liciu F. Nucleolar behavior in regenerating liver of rats receiving intraabdominal injections of azo dyes and thioacetamide. J Natl Canc Inst. 1968;40:917–933. [PubMed] [Google Scholar]
- 16.Alliegro MC, Alliegro MA. Centrosomal RNA correlates with intron-poor nuclear genes in Spisula oocytes. Proc Natl Acad Sci USA. 2008;105:6993–6997. doi: 10.1073/pnas.0802293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Love R, Walsh RJ. Nucleolinar morphology in normal diploid, neoplastic and aneuploid cells in vitro. Cancer Res. 1968;30:990–997. [PubMed] [Google Scholar]


