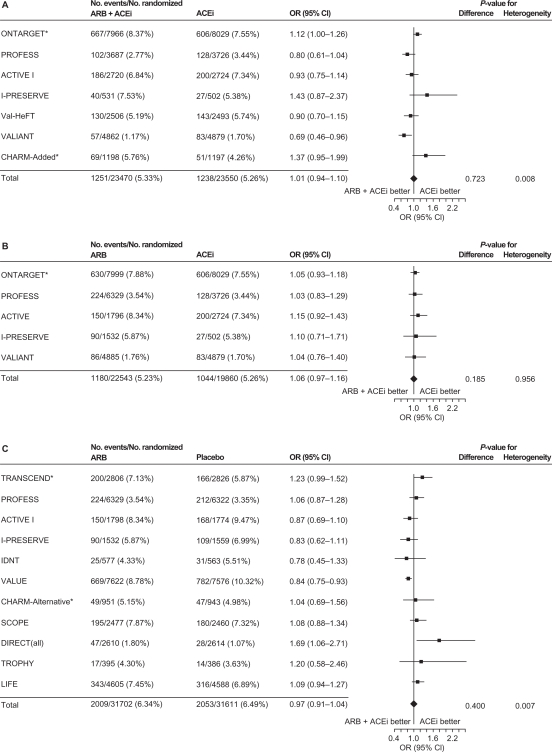
Figure 3.
Incidence of cancer with A) ARB/ACE inhibitor combination vs ACE inhibitor alone, B) ARB alone vs ACE inhibitor alone, and C) ARB vs placebo/control with no ACE inhibitor.
Reprinted from the Journal of Hypertension, volume 29, issue 4, the ARB trialists collaboration, ‘Effects of telmisartan, irbesartan, candesartan, and losartan on cancers in 15 trials enrolling 138 769 individuals’, pp 623–635, Copyright 2011, with permission from Wolters Kluwer Health.57
Notes: In the LIFE study, atenolol was the control. *Included patients who were free of cancer at baseline.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ACTIVE, Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events; ARB, angiotensin receptor blocker; CHARM, Candesartan in Heart failure Assessment in Reduction of Mortality; CI, confidence interval; DIRECT, Diabetic Retinopathy Candesartan Trials; IDNT, Irbesartan in Diabetic Nephropathy Trial; I-PRESERVE, Irbesartan in Heart Failure With Preserved Systolic Function; LIFE, Losartan Intervention For Endpoint Reduction in Hypertension; ONTARGET, Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial; OR, odds ratio; PROFESS, The Prevention Regimen for Effectively Avoiding Second Strokes Trial; SCOPE, Study on Cognition and Prognosis in the Elderly; TRANSCEND, Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease; TROPHY, Trial of Prevention of Hypertension; Val-HeFT, Valsartan Heart Failure Trial; VALIANT, Valsartan in Acute Myocardial Infarction; VALUE, Valsartan Antihypertensive Long-Term Use Evaluation.