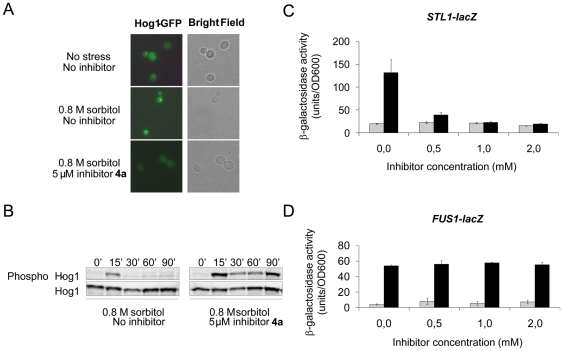
Figure 7. In vivo activity and selectivity of inhibitor 4a.
(A) Nuclear accumulation of Hog1 is prevented in the presence of 4a. A plasmid encoding a Hog1-GFP fusion protein was transformed into the hog1Δ mutant, and living cells were analyzed by fluorescence microscopy for Hog1 localization. Cells were either untreated or exposed to osmotic stress (0.8 M sorbitol). Inhibitor (5 µM) was added to cells 15 minutes before osmotic stress was applied. (B) Hog1 dephosphorylation is prevented in the presence of 4a. Hog1 phosphorylation was monitored in cells exposed to osmotic stress (0.8 M sorbitol) by Western blot analysis using an antibody specific to dually phosphorylated p38 MAPK, and an anti-Hog1 antibody was used as a control. Inhibitor (5 µM) was added to cells 15 minutes before osmotic stress was applied. (C) Inhibition of Hog1-dependent gene expression. Exponentially growing cells harboring the STL1-lacZ reporter were exposed to osmotic stress (0.8 M sorbitol) and assayed for β-galactosidase activity as described in the Experimental section. Induced expression of the STL1 gene by osmotic stress required Hog1 but no other signal transduction pathways. Inhibitor was added to cells at the indicated concentrations 10 minutes before osmotic stress was applied. The results are the average of three independent experiments and the error bars represent standard deviation (s.d.). (D) 4a is selective for Hog1 inhibition since it does not affect the Fus3/Kss1 MAPKs. Exponentially growing cells harboring the FUS1-lacZ reporter were exposed to α-factor (10 µM) and assayed for β-galactosidase activity as described above. Induced expression of the FUS1 gene in response to α-factor required Fus3 and Kss1 but was independent of Hog1 [38].