Abstract
Patients with childhood absence epilepsy (CAE) often demonstrate impaired interictal attention, even with control of their seizures. No previous study has investigated the brain networks involved in this impairment. We used the Continuous Performance Task (CPT) of attentional vigilance and the Repetitive Tapping Task (RTT), a control motor task, to examine interictal attention in 26 children with CAE and 22 matched healthy controls. Each subject underwent simultaneous 3T functional magnetic resonance imaging-electroencephalography (fMRI-EEG) and CPT/RTT testing. Areas of activation on fMRI during the CPT task were correlated with behavioral performance and used as seed regions for resting functional connectivity analysis. All behavioral measures reflecting inattention were significantly higher in patients. Correlation analysis revealed that impairment on all measures of inattention on the CPT task was associated with decreased medial frontal cortex (MFC) activation during CPT. In addition, analysis of resting functional connectivity revealed an overall decrease within an ‘attention network’ in patients relative to controls. Patients demonstrated significantly impaired connectivity between the right anterior insula/frontal operculum (In/FO) and MFC relative to controls. Our results suggest that there is impaired function in an attention network comprising anterior In/FO and MFC in patients with CAE. These findings provide an anatomical and functional basis for impaired interictal attention in CAE, which may allow the development of improved treatments targeted at these networks.
Keywords: epilepsy, attention, fMRI, networks, connectivity
Introduction
Childhood absence epilepsy (CAE) is a common form of pediatric epilepsy, accounting for 10–17% of all cases of epilepsy diagnosed in school-aged children (Berg et al., 2000; Jallon et al., 2001). Seizures are characterized by 3–10 second episodes of impaired consciousness without major motor symptoms, accompanied by bilateral (generalized) 3–4 Hz spike-and-wave discharges (SWD) on electroencephalogram (EEG). It has long been known that children with epilepsy, particularly CAE, suffer from attention problems between seizures (Caplan et al., 2008; Dunn et al., 2003; Henkin et al., 2005; Holdsworth and Whitmore, 1974; Levav et al., 2002; Mitchell et al., 1992; Sherman et al., 2007; Vega et al., 2010), and that these impairments often persist with treatment of seizures (Williams et al., 2002) as recently demonstrated in an important study (Glauser et al., 2010). The disease can have a significant impact on children’s lives, with increased requirements for special education, more frequently repeated grades, and a higher incidence of below-average school performance (Wirrell et al., 1997). These problems can persist into adulthood with lower level of employment and more social isolation (Olsson and Campenhausen, 1993).
Advances in neuroimaging have led to a better understanding of the brain networks involved in absence seizures that correspond to SWD and impaired ictal attention (Blumenfeld, 2005). Early techniques combined simultaneous EEG with fluoro-2-deoxy-D-glucose positron emission tomography (Engel et al., 1985; Theodore et al., 1985), single photon emission computed tomography (Iannetti et al., 2001; Kapucu et al., 2003; Yeni et al., 2000), and more recently blood-oxygen-level dependent functional magnetic imaging (BOLD fMRI) (Aghakhani et al., 2004; Archer et al., 2003; Bai et al., 2010; Berman et al., 2010; Gotman et al., 2005; Hamandi et al., 2006; Labate et al., 2005; Laufs et al., 2006; Li et al., 2009; Moeller et al., 2008a; Moeller et al., 2008b; Salek-Haddadi et al., 2003). To our knowledge, there is only one published study that correlated CAE interictal brain function with behavior (Duncan et al., 2009) in which investigators examined event-related brain potentials during auditory and visual tasks of attentional vigilance, and demonstrated a more profound reduction in the auditory P300 amplitude. No study to date has investigated the attention networks that may be disrupted in CAE during the interictal period.
Resting functional connectivity is a powerful tool for analyzing brain networks by temporally correlating activity in remote cortical and subcortical regions at rest. It was first used in fMRI to reveal brain regions associated with motor function oscillating in synchrony with the pre-central hand region, thereby revealing a ‘motor network’ (Biswal et al., 1995). Recent fMRI studies have demonstrated altered resting functional connectivity in temporal lobe epilepsy (Bettus et al., 2009; Liao et al., 2010; Morgan et al., 2010; Waites et al., 2006; Zhang et al., 2009a) including impaired attention networks (Zhang et al., 2009b). Another study established that this disruption may be more profound in generalized than partial seizures (Lui et al., 2008).
In the present study, we have demonstrated impaired interictal attentional vigilance in CAE patients relative to matched controls using the continuous performance task (CPT) with simultaneous EEG and fMRI. CPT is a test of sustained attention first developed to measure “brain damage” (Beck et al., 1956), and has been used subsequently to investigate interictal attention in CAE (Fedio and Mirsky, 1969; Mirsky et al., 1960). We used fMRI task activations to define an attention network and determine whether there were alterations within this network in patients.
Methods
Subjects
All human subject procedures were approved by the institutional review boards at Yale University School of Medicine, and by the Yale Magnetic Resonance Imaging Center. Children between 6–19 years of age with a history of typical childhood absence epilepsy and age/gender matched healthy controls participated in this study after written informed consent. As described previously (Bai et al., 2010; Berman et al., 2010), patients were referred by their pediatric neurologists and fulfilled the following inclusion criteria: a) clinical diagnosis of CAE based on International League Against Epilepsy criteria (ILAE, 1989), b) EEG with typical 3–4 Hz bilateral spike-wave discharges and normal background activity; and exclusion criteria: a) any history of additional seizure types (e.g. myoclonic, tonic-clonic or partial seizures), and b) any structural brain abnormality or any other neurological disorder. All normal controls were recruited locally using newspaper, internet and flyer postings, and whenever possible we recruited the patients’ unaffected siblings or friends from the same demographic group.
Prior to study participation, subjects were familiarized with the MRI environment and sounds in a mock scanner, and underwent a practice session with each of the behavioral tests. To facilitate ongoing studies on ictal behavior (Bai et al., 2010; Berman et al., 2010), patients who were on seizure medication were tested after holding their medication for up to 48 hours. This procedure was approved by our human studies institutional review board and was successfully implemented with no adverse side effects. The specifics of our medication-withholding protocol has been described previously (Berman et al., 2010).
Subjects’ and parents’ (generally maternal) intelligence quotients (IQs) were obtained with the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) using the estimated full scale IQ, which includes a verbal and nonverbal subtest. Socioeconomic status was quantified with the Hollingshead Four Factor Scale (Hollingshead, 1976), which uses educational and occupational information to obtain a value ranging from 8 to 66. Both tests were scored by a neuropsychologist (CV or MS) and used along with age, gender and handedness to match cohorts of patients and controls. This was accomplished while blinded to subjects’ identity, behavioral performance, and EEG or fMRI data.
Behavioral tasks and analysis
Two behavioral tasks and a visual fixation task were performed during the EEG-fMRI acquisitions. Although many of subjects performed all three tasks, task order across subjects was random, and several performed only one or two of the tasks due to variable ability of the children to tolerate longer scanning sessions (the number and demographics of subjects performing each task are summarized in Table 1 and Supplementary Table 1). The behavioral tasks have been employed previously (Bai et al., 2010; Berman et al., 2010; Jus and Jus, 1960; Mirsky and Buren, 1965; Yeager and Guerrant, 1957) to examine the impairment of consciousness in patients with typical absence epilepsy. All tasks were generated using E-Prime 1.1 (Psychology Software Tools, Inc. Pittsburgh, PA). Each fMRI task run consisted of twenty 32 second blocks alternating between 1 Hz letter displays and a visual fixation cross (total run duration 640 seconds). During the continuous performance task (CPT) 16 letters were displayed for 250ms in a randomized sequence (e.g. A B C D E F H I L M N O T X Y Z). Twenty-five percent of all letters shown were the target X, and patients were instructed to respond to the target letter X by using their right thumb to push a button. The repetitive tapping task (RTT) was the same as the CPT except subjects were instructed to push the button for every displayed letter and no letter X appeared in the sequence. The visual fixation task consisted of twenty uniform fixation blocks (run duration 640 seconds). For all tasks, the letters and fixation cross appeared on a rear-projection screen, viewed by a mirror mounted on the patient head coil. Both the stimuli and button push response times for displayed letters were recorded using the E-Prime program.
Table 1.
Subject demographics and behavioral performance on the CPT task.
| Patients | Controls | p value | |
|---|---|---|---|
| Demographics | |||
| N | 26 | 22 | |
| Age (y) | 12 ± 4 | 12 ± 3 | NS |
| gender (f/m) | 15/11 | 13/9 | NS |
| handedness (r/l) | 21/5 | 20/2 | NS |
| child IQ | 105 ± 12 | 110 ± 15 | NS |
| parent IQ | 109 ± 12 | 109 ± 18 | NS |
| Hollingshead | 48 ± 11 | 52 ± 10 | NS |
| CPT performance | |||
| omission rate (%) | 5.1 ± 5.1 | 2.1 ± 3.4 | 0.021 |
| comission rate (%) | 2.3 ± 5.1 | 1.1 ± 1.3 | NS |
| mean reaction time (ms) | 513 ± 55 | 461 ± 75 | 0.010 |
| st. dev. reaction time (ms) | 106 ± 24 | 86 ± 24 | 0.007 |
| beta | 4.4 ± 4.5 | 2.4 ± 3.6 | NS |
| d-prime | 4.2 ± 0.7 | 4.7 ± 0.8 | 0.017 |
All values are mean ± standard deviation.
For data analysis, the intervals for correct responses on the CPT task were 120–1000ms after stimulus presentation. Performance on the task was analyzed for all subjects using in-house code written on a MATLAB 7.1 platform (MathWorks, Natick, MA) and included omission rate (number of missed targets/total targets), commission rate (number of button pushes to nontargets/total nontargets), reaction time, and standard deviation of reaction time (as a measure of within-subject reaction time variability). We also calculated the signal detection theory measures d-prime (“sensitivity,” or the difference between signal and noise means along the decision variable axis) and beta (“response bias” or general tendency to push or not push the response button) (Stanislaw and Todorov, 1999; Wickens, 2002) as these measures have been used in prior behavioral studies of the CPT task (Buchanan et al., 2010; Conners et al., 2003; Epstein et al., 2003).
Any behavioral data acquired during spike-wave episodes based on EEG were excluded from analysis. Blocks during which subjects did not participate, i.e. no button pushes during the entire block due to drowsiness or sleep (and were subsequently determined not to be seizing), were not counted in analysis of performance and not modeled with task during fMRI analysis. In addition, if 5 or more blocks were thus excluded from any single run, the entire run was discarded.
EEG-fMRI Data acquisition
Continuous EEG-fMRI data were acquired from each patient to enable us to remove any spike-wave discharges from the analysis and to focus on the baseline interictal state without seizure activity. EEG data were acquired with two slightly different systems. During the first half of the study (12 of 26 patients), data were collected by an EEG cap with silver/silver-chloride electrodes (modified from Quik-Cap 21 channel (international 10–20 system), Neuroscan Inc., North Carolina, U.S.A.), carbon fiber cables (in-house), a 125Hz analog low-pass Butterworth filter (in-house), and an EEG recorder (NuAmps, Neuroscan Inc.). EEG signals were recorded at a 500 Hz sampling rate with 32-bit data resolution and referred to a reference electrode between Fz and Cz. The EEG data in the remaining patients were recorded using the same system except that 32 carbon wire EEG electrodes (in-house) and a pre-amplifier (in-house) were used (Negishi et al., 2008), and the signals were digitized at 1000Hz with a newer EEG recorder (SynAmps2, Neuroscan Inc.). In controls, identical EEG setup and cap placement were used, and no EEG abnormalities were observed during a brief screening EEG obtained before entering the scanner. No EEG data were collected in controls during the scans although the cap remained in place to ensure the experience for patients and controls was identical.
All patients and controls were scanned using a 3 Tesla Magnetom Trio scanner (Siemens Medical Systems, Erlangen, Germany). During scanning, foam padding was used to help secure the EEG leads, reduce motion artifacts, and improve patient comfort. Prior to the task, AC-PC aligned axial T1 anatomical images (spin echo, repetition time = 300 ms, echo time = 2.47 ms, matrix size = 256 × 256, 25 slices per image, slice thickness = 6 mm, field of view = 22cm) were acquired in the same image planes as the functional MRI data. Functional images were acquired with an echo-planar imaging BOLD sequence (repetition time = 1550 ms, echo time = 30 ms, flip angle = 80, matrix size = 64 × 64, other parameters were the same as the T1 anatomical images).
BOLD fMRI was obtained in 640 second runs. At the beginning of each fMRI run, a transistor-transistor logic pulse from the MRI scanner was supplied to one EEG channel in order to accurately match the fMRI, EEG and behavioral task time series. To further ensure correct synchronization, the behavioral stimulus presentations and button push responses were recorded along with the EEG data in separate channels, as well as by the stimulus presentation software. Up to 6 (typically 3 or 4) fMRI runs were obtained per recording session as tolerated by the patients.
EEG analysis
EEG data were filtered offline to remove the artifact generated by MRI scans, allowing the visualization of the entire EEG trace. The MR artifact in the first 12 patients was filtered using the SCAN (Neuroscan) software and in-house temporal principal component analysis (PCA) software (Negishi et al., 2004). The MR artifact in the remaining patients was subtracted using adaptive noise cancellation software (Negishi et al., 2008). After artifact removal, the EEG data were low-pass filtered at 50 Hz and visually inspected for typical 3–4 Hz spike-wave discharges. The onset and offset times of spike-wave discharges were identified by an epilepsy neurologist (HB). All identified spike-wave discharges were considered epileptiform abnormalities regardless of duration, or clinical behavioral change, and were removed from the analysis.
fMRI analysis
The SPM2 software package (http://www.fil.ion.ucl.ac.uk/SPM) was used for all fMRI preprocessing on a MATLAB platform. The initial 10 images (15.5 seconds) were discarded in each run. The remaining 406 images in each run were spatially realigned to the first image of each functional series, using 3D rigid body (linear) transformation with three translation and three rotation parameters to minimize the mean squared difference between the images as implemented in the SPM software (Woods et al., 1998). Images were then spatially normalized to the SPM EPI template in MNI space. Images were then spatially smoothed using an isotropic Gaussian kernel (10-mm full width at half maximum).
Signal to noise ratio (SNR) was calculated for each fMRI run using in-house code written in MATLAB. For this calculation, the SPM2 whole brain mask (brainmask.nii) was applied to all warped images prior to spatial smoothing, and the time course mean BOLD signal was calculated at each voxel. SNR was defined as 20*log(mean BOLD signal/standard deviation of BOLD signal), and a whole brain mean SNR was calculated for each fMRI run. An arbitrary cut-off of 30 dB was set, and all runs with SNR below this were excluded from analysis.
Statistical analysis of fMRI data was performed with the general linear model approach in SPM2. For each subject, variance due to movement was removed from the data through linear regression by including the six parameters obtained by rigid body correction of head motion in the statistical model. Epochs of task blocks and spike-wave discharges were used to construct boxcar models of task versus fixation and spike-wave versus baseline EEG state, which were convolved with the canonical hemodynamic response function from SPM2 to form the two primary regressors. Both the fMRI data and the design matrices were then high pass filtered at 128s, and the resulting model was pre-whitened by an autocorrelation AR(1) model. No global scaling was used in the analysis.
Single-subject analyses were performed first to determine fMRI changes during task vs. fixation blocks using a t-contrast in SPM2. In patients who had spike-wave episodes during task performance, we included the spike-wave events as a regressor in the model to remove their effects from the fMRI results. Analysis of spike-wave-related changes are described elsewhere (Bai et al., 2010; Berman et al., 2010). For group analyses, a one-sample t-test using a random effects model was performed in SPM2 (Holmes and Friston, 1998) to determine regions showing significant fMRI changes across subjects during task vs. fixation blocks. Separate analyses were performed for the CPT and the RTT tasks. We applied a family wise error (FWE)-corrected height threshold p = 0.05 to correct for multiple comparisons with an extent threshold of k = 3 voxels (voxel dimensions = 4 mm × 4 mm × 4 mm). The FWE correction in SPM is based on Gaussian random field theory and takes into account the likelihood that a family of voxel values could arise by chance alone (Friston et al., 1996). All results for functional data were displayed superimposed on the Montreal Neurological Institute brain template “colin27” (single_subj_T1 in SPM) in radiological convention, using a MarsBaR (http://marsbar.sourceforge.net/) mask to display only gray matter voxels.
Attention regions of interest were next determined by areas of activation surviving a two sample t-test comparing CPT activations versus RTT activations across all subjects (uncorrected p threshold = 0.01, extent threshold k=3 voxels). The resultant three attention ROIs were used to extract mean beta values corresponding to task activation (obtained from the statistical model and related to the regressor’s contribution to the overall time series) for each CPT run. The beta values are therefore related to the strength of fMRI activation during task. In-house code was written in MATLAB to extract beta values at each voxel and to then calculate a mean and standard deviation within each ROI. These values were used to run a linear regression analyses in SPSS (SPSS Inc., Chicago, IL) against the attentional target detection values from subjects’ CPT performance.
Resting functional connectivity
Fixation only runs were used for connectivity analysis. The first 33 acquisitions in each run were discarded to ensure participants were under the resting state. All epochs of movement greater than 1 mm translation or 1 degree of rotation were discarded. Images from −40s before to +60s after each seizure onset were discarded to avoid the effect of BOLD changes due to seizures (Bai et al., 2010). The longest remaining interictal epoch for each run was matched one-to-one for each patient and control such that the number of runs and time segments within the runs were identical. Resting functional connectivity analysis was performed using in-house code written in MATLAB. Two sources of erroneous variance were removed through linear regression and subtraction from the data: (i) six parameters obtained in the realignment preprocessing step by rigid body correction of head motion and (ii) the signal average for each slice. The data were next band-pass filtered (0.01 < f < 0.08 Hz) to remove low-frequency drift and reduce the influence of high-frequency noise.
For resting functional connectivity analysis we used the three attention ROIs obtained as described above. For individual subjects, a mean timecourse was calculated by averaging the timecourses of each voxel within the seed ROI. A correlation map was then produced by computing the Pearson’s correlation coefficient between mean time course for each ROI and the time course from all brain voxels. Subsequently, this correlation map was converted to z-scores at each voxel by Fisher’s z transform z(r) = 0.5ln[(1+r)/(1−r)] (Jenkins and Watts, 1968). To normalize for differences in number of images, each z score was divided by the square root of variance, calculated as , where n is the degrees of freedom defined as the number of image acquisitions within each epoch. For participants with multiple fixation runs, we averaged the z-score maps across runs. Analysis was confined to the gray matter by applying a standard gray matter mask from MarsBaR (http://marsbar.sourceforge.net/) to all z-score maps. For each of the three ROIs, we next ran a single sample t-test in SPM across all subjects (patients and controls combined) to show voxels significantly correlated with that ROI in the whole brain.
The z-score maps generated in this way were next used to compare patients and controls using an ROI-based analysis. Using each ROI as the reference, we calculated the mean z-values in the other two ROIs in all subjects. This resulted in three pairs of reciprocal connections, which were then averaged to obtain a single value for each pair within each subject. For group statistical analysis, we performed one-way ANOVA followed by Tukey’s HSD method for post-hoc pairwise comparison to assess the differences in nodal connections between the patient and control groups.
The three attention ROIs used for this analysis were based on our block-design task above, and were very similar (see Results) to medial frontal/anterior cingulate and insular/frontal opercular regions described previously for sustained attention (Nomura et al., 2010). As an additional exploratory approach, we expanded the network analysis to include additional fronto-parietal regions involved in transient attention based on previously published work. Thus, using the same resting functional connectivity methods we studied the bilateral intraparietal sulcus (right: 30, −61, 39; left: −31, −59, 42), frontal cortex (right: 41, 3, 36; left: −41, 3, 36), precuneus (right: 10, −69, 39; left: −9, −72, 37), lateral parietal cortex (right: 51, −47, 42; left: −51, −51, 36), dorsolateral prefrontal cortex (right: 43, 22, 34; left: −43, 22, 34), and midcingulate (0, −29, 30), all using spherical ROIs in locations as published previously (Nomura et al., 2010).
Results
Impaired attention in CAE patients vs. matched controls
A total of 26 patients and 22 controls matched for age, gender, handedness, IQ (subjects’ and maternal), and socioeconomic status performed the continuous performance task (CPT) (Table 1). Demographics for patients and controls from the overlapping groups who performed the motor control repetitive tapping task (RTT), and who performed fixation runs for resting functional connectivity analysis were also well matched (see Supplementary Table 1).
Patients’ impaired interictal attention relative to controls was apparent in their execution of the continuous performance task. Signal detection parameters reflecting inattention were significantly impaired in patients: higher omission rates (p=0.021; two-tailed t-test), longer (p=0.010) and more variable (p=0.007) reaction times, and lower d-prime values (p=0.017). No significant differences were found between groups for indexes of impulsivity: commission rates (p=0.34) or beta values (p=0.10) for signal detection. The subjects’ task performance results are summarized in Table 1.
Decreased attention performance is related to reduced fMRI signal in MFC
To investigate brain regions involved in attention performance, we first evaluated fMRI changes during tasks in the entire study subject population. BOLD increases above fixation during the continuous performance task (Figure 1A) included areas involved in attention networks described in previous publications (Dosenbach et al., 2007; Dosenbach et al., 2006; Eckert et al., 2009; Giraud et al., 2004; Sridharan et al., 2008; Thielscher and Pessoa, 2007): the bilateral anterior insulae and frontal opercula, medial frontal cortex comprised primarily of the supplemental motor area (SMA) and anterior cingulate cortex (ACC), as well as the upper extremity portion of the left motor cortex. The repetitive tapping task more strongly activated motor networks, with BOLD increases in the upper extremity portion of the left motor cortex, the SMA, and the inferior right parietal lobe (see Supplemental Figure 1).
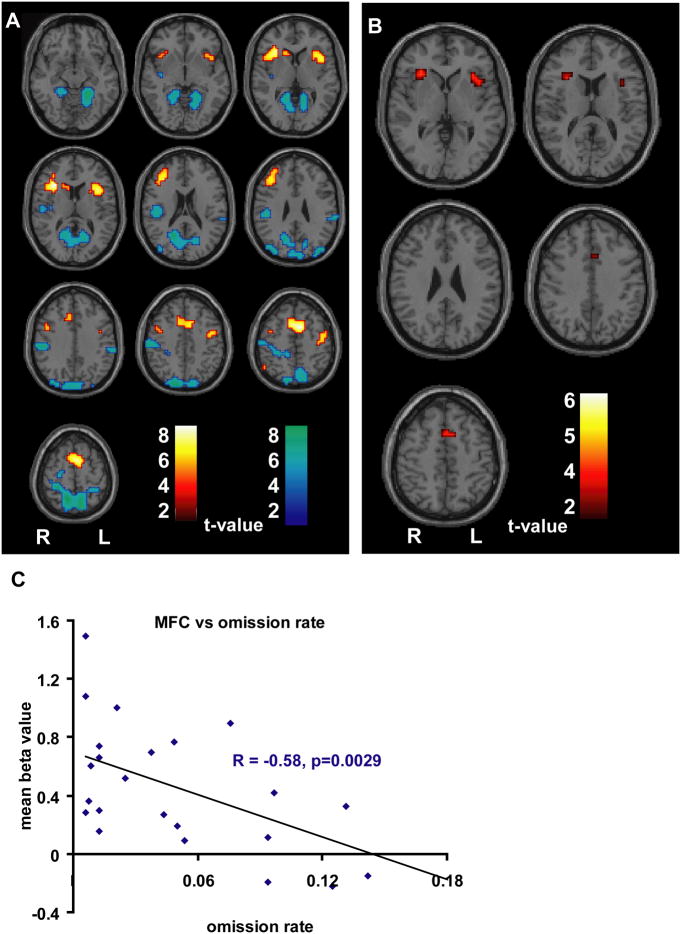
Figure 1.
BOLD fMRI changes during the CPT task. A. t map demonstrating increases (hot colors) and decreases (cold colors) during CPT (N=48; 26 patients, 22 controls) relative to fixation (height threshold p<0.05, FWE corrected, extent threshold k=3 voxels). Significant fMRI increases included the medial frontal cortex (MFC) comprised of anterior cingulate cortex (ACC) and supplemental motor area (SMA), bilateral insulae/frontal opercula (In/FO), and upper extremity portion of the left motor cortex. B. Results of 2-sample t test showing regions with greater fMRI increases on CPT than on RTT (height threshold p<0.01 uncorrected for multiple comparisons, extent threshold k=3 voxels) excluding areas not activated during the CPT task (A). Three presumed attention-related regions were identified as MFC and bilateral In/FO, which were used as ROIs in subsequent analyses. For regions activated on RTT see Supplementary Figure 1. C. Correlation analyses between CAE patients’ omission rate and mean beta values within the medial frontal cortex ROI during CPT (n=26). Declining performance, as evidenced by increased omission errors, was correlated with declining beta values in MFC. Similar results for reaction time, standard deviation of reaction time, and d-prime are shown in Supplemental Figure 2.
To more selectively investigate the regions involved in attention rather than motor function, we next performed an exploratory analysis to remove RTT increases from CPT increases, which we posited would more effectively isolate the attention areas. To accomplish this, we performed a two-sample whole-brain t-test between all subjects’ CPT and RTT activations. Moreover, to ensure that the resulting analysis yielded regions activated during CPT, we excluded regions that were sub-threshold in the prior one-sample t-test of CPT activations (Figure 1A). The resulting t-map is displayed in Figure 1B and demonstrates three regions: the bilateral anterior insula/frontal operculum (In/FO) and a portion of the medial frontal cortex (MFC) at the junction of the ACC and the inferior SMA. While the results of this second-level analysis were exploratory and did not survive correction for multiple comparisons, this procedure provided more selective attention-related ROIs for subsequent analyses.
We next examined how the identified ROIs related to attention impairment in children with CAE by performing correlation analyses. Regardless of the index of attention (omission rate, reaction time, standard deviation of reaction time, d-prime), impaired patient attention performance correlated significantly with decreased mean beta values within the medial frontal ROI. That is, worse attention in patients was associated with decreased BOLD fMRI signal within the medial frontal lobes during CPT. No significant correlations existed between the other two ROIs (left and right In/FO) and attention performance, nor were any of the three ROIs correlated significantly with scores on the impulsivity parameters (commission rate and beta). Figure 1C demonstrates the inverse relationship between patients’ medial frontal activation and CPT omission rate (R = −0.58, p = 0.0029, 2-tailed R to t conversion). Correlations of the medial frontal cortex with other behavioral measures are displayed in Supplemental Figure 2. Similar patterns of correlation were seen in controls, and no significant differences were found between the two groups when compared directly (data not shown).
CAE patients have impaired resting functional connectivity between MFC and right In/FO
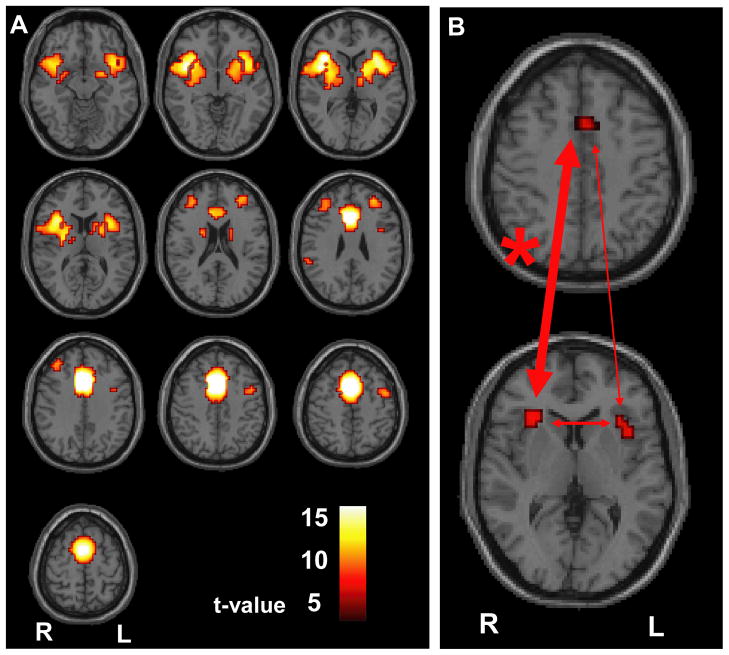
The above findings demonstrate that reduced attention performance was related to decreased activation of MFC. However, this was true for both patients and controls. Since we were interested in fundamental differences that may explain the overall worse performance in patients, we next eliminated effects of task performance by studying the attention network at rest. We used each of the three attention ROIs (Figure 1B) as seed regions for whole brain voxel-wise correlation resting functional connectivity analysis. We found strong correlations that were maximal between each attention ROI and the other two ROIs (Figure 2A; See also Supplemental Figure 3). This finding validated our defining a functional network involving the bilateral In/FO and MFC, which was related to CPT activations and also showed strong interconnectivity at rest.
Figure 2.
Resting functional connectivity analysis. A. Medial frontal ROI seed region shows maximal connectivity with bilateral anterior insula/frontal operculum, paralleling the network activated during the CPT task. t map with n = 32 (16 patients, 16 controls), height threshold was made more stringent at p<0.001, FWE corrected to reveal connectivity maxima, extent threshold k=3 voxels. B. Results of ANOVA comparing patient and control connectivity between attention ROIs. Mean z-score for connectivity between right In/FO and MFC was 5.4 ± 2.0 (mean ± SD) for patients and 7.2 ± 1.9 for controls (p = 0.016); between left In/FO and MFC was 6.5 ± 2.5 for patients and 7.5 ± 2.9 for controls (p = 0.29); and between left In/FO and right In/FO was 7.3 ± 3.0 for patients and 8.9 ± 3.3 for controls (p = 0.16). Widths of arrows are proportional to F values. * = p<0.05.
We were next interested in determining whether resting connectivity in this network differed between patients and controls. We found that all connections between the MFC, and right and left In/FO were generally weaker in CAE patients relative to controls, and this difference reached statistical significance for the connectivity between the right In/FO and MFC (Figure 2B). Mean connectivity z-score between right In/FO and MFC was 7.2 ± 1.9 (mean ± SD) in controls but only 5.4 ± 2.0 in patients (p=0.016). This finding reveals abnormal resting functional connectivity between the medial frontal cortex and the right insula/frontal operculum in patients with CAE, suggesting that there is a fundamental deficit in attention network function in these patients.
Although subgroup analyses should be interpreted with caution, particularly when sample size is limited, we performed a preliminary analysis on a subset of CAE patients and controls that were matched for impaired attention performance (CAE patients and controls respectively, omission error rate = 8.0 ± 10.2 % and 7.7 ± 8.8 % (mean ± SD); mean age = 10 ± 4.3 and 10 ± 2.8 years; n = 6 (all female) and 6 (5 female)). In these subgroups we still found a reduction in mean connectivity z-score between right In/FO and MFC in patients (5.6 ± 2.4 (mean ± SD)) compared to controls (8.0 ± 1.0; p=0.047), suggesting the reduced connectivity in CAE patients is not a non-specific effect of attention performance. As a further exploratory analysis, we also investigated resting connectivity in a broader attention network including more fronto-parietal ROIs taken from prior work (Nomura et al., 2010) but did not find additional connections with significant connectivity differences between patients and controls. This further supports the importance of the MFC - In/FO network for impaired attention in childhood absence epilepsy.
Discussion
CAE patients demonstrated significant interictal impairment relative to matched controls on behavioral testing, and this impairment was reflected in disruption of their attention network. Based on fMRI activations, attention ROIs included the bilateral anterior insulae/frontal opercula (In/FO) and medial frontal cortex (MFC). Correlation analysis revealed that impairment in all behavioral measures of attention was associated with decreased medial frontal fMRI activation during CPT. Analysis of resting functional connectivity confirmed the existence of an attention network and demonstrated decreased connectivity in patients relative to controls within this network. These findings provide for the first time an anatomical and functional basis for impaired interictal attention in CAE.
Previous publications have addressed many of the components of this study. It is well established that children with CAE suffer from interictal attentional impairment (Caplan et al., 2008; Dunn et al., 2003; Henkin et al., 2005; Holdsworth and Whitmore, 1974; Levav et al., 2002; Mitchell et al., 1992; Sherman et al., 2007; Vega et al., 2010), and that this dysfunction often persist with treatment of seizures (Williams et al., 2002). This was recently investigated in a landmark study by the Childhood Absence Epilepsy Study Group (Glauser et al., 2010) that demonstrated impaired performance on the Conners continuous performance test in children with CAE both before treatment and on three different medication regimens. None of these studies sought to investigate the underlying mechanism of this impairment.
Recent fMRI research has demonstrated altered resting functional connectivity in temporal lobe epilepsy (Bettus et al., 2009; Liao et al., 2010; Morgan et al., 2010; Waites et al., 2006; Zhang et al., 2009a) including impaired attention networks (Zhang et al., 2009b). Another study established disrupted connectivity involving the default mode that was more profound in epilepsy patients with generalized than partial seizures (Lui et al., 2008). No publication to date has analyzed these networks in the CAE population, though there is indirect evidence of their disruption. Previous research has demonstrated selective network involvement in generalized seizures (Bai et al., 2010; Blumenfeld et al., 2009; Blumenfeld et al., 2003; Meeren et al., 2002; Meeren et al., 2005; Nersesyan et al., 2004), and there is structural MRI, MR spectroscopic, and diffusion tensor imaging data in animal models (Chahboune et al., 2009) and human subjects (Chan et al., 2006; Fojtiková et al., 2006) that suggest disruption of similar cortical and subcortical networks in CAE that persists between seizures. Moreover, there is evidence that impaired behavioral performance is reflected in reduced resting functional connectivity between areas activated during behavioral tasks across a variety of neurological disorders (Agam et al., 2010; Hampson et al., 2010; Hampson et al., 2006; Tu et al., 2010; Wang et al., 2010), though this has never been examined in CAE. This line of evidence directed us to investigate network disruption in CAE underlying attentional impairment.
We found that activation of the MFC (comprised largely of the ACC) was central in sustaining attention during CPT. This is consistent with electrophysiological studies spanning three decades demonstrating involvement of the MFC in focusing attention and modulating target selection (Posner et al., 1988) as well as resolution of conflict errors and monitoring performance by the executive attention system (Gehring and Knight, 2000; Luu et al., 2000). More recent fMRI studies have confirmed these results, demonstrating ACC activation in error detection (Carter et al., 1998), executive control of attention (Fan et al., 2005a), and conflict monitoring and conflict control (Kerns et al., 2004). Moreover, there is evidence that ACC activation occurs with either manual or vocal output and spatial or verbal processing (Barch et al., 2001). One recent fMRI study of sustained attention demonstrated bilateral insulae and ACC activation, and correlation of worse performance with less BOLD signal of the latter (Tana et al., 2010), a finding we replicated in our CAE population. Other studies have demonstrated that bilateral insulae activations may be associated with assessment of risk (Knutson and Bossaerts, 2007), negative reward anticipation (Liu et al., 2007), and impulse control (Knutson et al., 2007).
The attention network that we identified in our study has been described previously in a diversity of attentionally taxing contexts. Giraud and colleagues employed an auditory speech task, and found that in addition to speech areas, auditory attention and comprehension was localized in the bilateral insulae, ACC and right medial frontal cortex (Giraud et al., 2004). Dosenbach (Dosenbach et al., 2006) using a mixed block/event-related design involving ten different tasks found that the dorsal ACC/medial superior frontal and bilateral anterior In/FO formed a “core” task-set system. Thielscher and Pessoa utilized a fear-disgust 2-choice discrimination task and concluded that ACC/MFC and In/FO activation correlated with decision making reaction time (Thielscher and Pessoa, 2007). Finally, Dosenbach and colleagues applied graph theory to resting functional fMRI data to propose a distinct task-control nework involving the dorsal ACC/MFC and anterior In/FO that may control goal-directed behavior through stable maintenance of task sets (Dosenbach et al., 2007).
Several studies have described distinct dorsal and ventral attention networks (Corbetta and Shulman, 2002; Fox et al., 2006; Kincade et al., 2005; Seeley et al., 2007) wherein task-specific signals from the dorsal system filter stimulus signals from the ventral system, and stimulus-driven “circuit-breaking” signals from the ventral system interrupt the dorsal system, thereby refocusing attention toward salient stimuli. The dorsal attention network is bilaterally represented and is involved in goal directed behavior and top-down orienting of attention (Corbetta et al., 2000; Corbetta et al., 2005; Hopfinger et al., 2000; Kastner et al., 1999; Kincade et al., 2005; Shulman et al., 2003; Slagter et al., 2007). The ventral attention system is right-hemisphere lateralized and functions to reorient attention in response to unexpected environmental stimuli (Astafiev et al., 2003; Corbetta et al., 2000; Kincade et al., 2005).
This right hemisphere dominance in attention has recently been carefully quantified (Shulman et al., 2010) and is reflected in our study’s establishing that CAE patients demonstrate impaired connectivity between the right anterior In/FO and MFC. In fact, a recent publication (Eckert et al., 2009) examined functional connectivity during several tasks of word recognition and spatial attention and found that the right anterior In/FO and ACC were engaged regardless of task stimulus presentation (auditory/visual) or mode of response (spoken/motor). They concluded that the right In/FO is the “heart of ventral attention system,” providing a link across attentional and task-supporting networks. These findings paralleled those of Sridharan and colleagues who demonstrated that a right In/FO - ACC network plays a major role in switching between distinct brain networks across task paradigms and stimulus modalities (Sridharan et al., 2008). These studies build a framework on which to interpret our results, and emphasize the significance of CAE patients’ impaired attention, both in terms of their behavioral performance and network connectivity.
Recent important studies using fMRI connectivity techniques to examine changes in brain attention control networks during development are also highly relevant to our findings. Multiple publications (Fair et al., 2009; Fair et al., 2007; Supekar et al., 2009) have demonstrated that during development there is an increase in long-range connections (‘integration’) and a decrease in short-range connections (‘segregation’) involved in control networks. Moreover, researchers have been able to apply this data to accurately predict subjects’ ages by analyzing the patterns of fMRI connectivity (Dosenbach et al., 2010). Another recent fMRI connectivity study (Kelly et al., 2009) examined the effect of age on networks involving the anterior cingulate cortex and demonstrated that connections were more local and diffuse in children and were more distant and discrete in adults when using the ACC as a seed region. In light of these findings, it is possible that the normal developmental processes of network segregation and integration are disrupted in children with CAE, which might account for differences we observed in our subjects. Further investigation of this possibility is warranted in future studies, and should also include new promising methods such as graph-theorerical analysis of network metrics and behavior (Fair et al., 2009; Fair et al., 2007). There are limitations to our study. CPT does not differentiate among particular attention networks, including orienting, alerting, and executive functions, as previously described (Fan et al., 2005a; Fan et al., 2005b; Fan and Posner, 2004). We realize that our “attention network” is an oversimplication - our aim was not to elaborate on subtleties of these networks, but rather to investigate the network disruption underlying CAE patients’ impaired interictal attention. Future studies using tasks that probe various aspects of attention networks will help to dissect the intricacies of this impairment. Performing EEG-fMRI and behavioral studies on young children is technically challenging, and though we included six motion-related regressors in our analyses, it is possible that the results could be improved further by inclusion of better models of motion effects (Lund et al., 2005). In addition, because the fixation task was added along with the other two tasks somewhat later in our study, the sample size and mean age of subjects performing the fixation task were slightly lower than in the other two groups (Table 1, Supplementary Table 1). Although the groups were generally overlapping and reasonably matched, it is possible that larger sample sizes in all groups will provide further insights. Finally, effects of medications cannot be fully excluded in our study. Although all patients were off medications for up to 48 hours at the time of testing, some residual effects of medications may have persisted. Ideally these results should be replicated in drug naïve patients or in patients off medications for more prolonged time periods.
In summary, CAE patients perform more poorly on a task of attentional vigilance, and the degree of this impairment correlates negatively with fMRI activation of the medial frontal cortex during task. Moreover, our fMRI results demonstrate impaired function in an attention network comprising the anterior insula/frontal operculum and medial frontal cortex, and this disruption is most marked between the right In/FO and MFC. These findings provide an anatomical and functional basis for interictal impaired attention in CAE, which may allow improved treatments to be developed targeted at these networks.
Supplementary Material
BOLD fMRI changes during the RTT task. A. t map demonstrating increases (hot colors) and decreases (cold colors) during RTT (N = 47; 25 patients, 22 controls) relative to fixation (height threshold p<0.05, FWE corrected, extent threshold k = 3 voxels). Significant fMRI increases included the upper extremity portion of the left motor cortex, the supplementary motor area, the right anterior insula, right frontal cortex, and right parietal cortex.
Correlation analyses between CAE patients’ attentional performance and mean beta values within the medial frontal ROI during CPT. Note that decreased medial frontal cortex (MFC) activation was correlated with all measures of worse CPT performance, including increased reaction time (A), increased standard deviation of reaction time (B), and decreased d-prime (C). Data are for same patients as shown for omission error rate in Figure 1C (n = 26).
Resting functional connectivity analysis for right and left In/FO seed regions. A. Left anterior insula/frontal operculum ROI seed region shows maximal connectivity with medial frontal cortex and right anterior insula/frontal operculum, B. Right anterior insula/frontal operculum ROI seed region shows maximal connectivity with medial frontal cortex and left anterior insula/frontal operculum, Note that findings parallel the attention network activated during the CPT task. t maps with n = 32 (16 patients, 16 controls), height threshold was made more stringent at p<0.001, FWE corrected to reveal connectivity maxima, extent threshold k = 3 voxels. Same data and subjects as in Figure 2.
Demographics of the RTT cohort and connectivity (fixation) cohort.
Acknowledgments
We are especially grateful to the patients and families who participated, and to the following clinicians who referred patients for the study: A Bhargava, H Blumenfeld, B Bourgeois, W Brown, G Castaneda, RL Cerciello, R Cheng, F DiMario, RB Duckrow, M Engel, J Gaitanis, J Gibbons, L Kan, SR Levy, D Mandelbaum, G Miller, S Moshe S Nallainathan, EJ Novotny, P Overby, S Rothman, R Smith, Y Sogawa, F Testa, S Wolf, and R Young. We also thank Michelle Hampson and Jennifer Roth for helpful discussion about the methods. This work was supported by NIH R01 NS055829 and by the Betsy and Jonathan Blattmachr family.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agam Y, Joseph R, Barton J, Manoach D. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 2010 Apr 13; doi: 10.1016/j.neuroimage.2010.04.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghakhani Y, Bagshaw A, Bénar C, Hawco C, Andermann F, Dubeau F, Gotman J. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127–1144. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- Archer J, Abbott D, Waites A, Jackson G. fMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1915–1922. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- Astafiev S, Shulman G, Stanley C, Snyder A, Essen DV, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, Desalvo M, Novotny EJ, Constable RT, Blumenfeld H. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. Journal of Neuroscience. 2010;30:5884–5893. doi: 10.1523/JNEUROSCI.5101-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D, Braver T, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Beck L, Bransome EJ, Mirsky A, Rosvold H, Sarason I. A continuous performance test of brain damage. J Consult Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Berg A, Shinnar S, Levy S, Testa F, Smith-Rapaport S, Beckerman B. How well can epilepsy syndromes be identified at diagnosis? A reassessment 2 years after initial diagnosis. Epilepsia. 2000;41:1269–1275. doi: 10.1111/j.1528-1157.2000.tb04604.x. [DOI] [PubMed] [Google Scholar]
- Berman R, Negishi M, Vestal M, Spann M, Chung M, Bai X, Purcaro M, Motelow J, Danielson N, Dix-Cooper L, Enev M, Novotny EJ, Constable RT, Blumenfeld H. EEG, fMRI, and behavior in typical childhood absence seizures. Epilepsia. 2010;51:2011–2022. doi: 10.1111/j.1528-1167.2010.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, Cozzone P, Chauvel P, Ranjeva J, Bartolomei F, Guye M. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp. 2009;30:1580–1591. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin F, Haughton V, Hyde J. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Consciousness and epilepsy: why are patients with absence seizures absent? Prog Brain Res. 2005;150:271–286. doi: 10.1016/S0079-6123(05)50020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese G, Purcaro M, Motelow J, Enev M, McNally K, Levin A, Hirsch L, Tikofsky R, Zubal I, Paige A, Spencer S. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Westerveld M, Ostroff R, Vanderhill S, Freeman J, Necochea A, Uranga P, Tanhehco T, Smith A, Seibyl J, Stokking R, Studholme C, Spencer S, IGIZ Selective frontal, parietal, and temporal networks in generalized seizures. Neuroimage. 2003;19:1556–1566. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- Buchanan R, Keefe R, Lieberman J, Barch D, Csernansky J, Goff D, Gold J, Green M, Jarskog L, Javitt D, Kimhy D, Kraus M, McEvoy J, Mesholam-Gately R, Seidman L, Ball M, McMahon R, Kern R, Robinson J, Marder S. A Randomized Clinical Trial of MK-0777 for the Treatment of Cognitive Impairments in People with Schizophrenia. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.09.052. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, Koh S, Sankar R, Shields W. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49:1838–1846. doi: 10.1111/j.1528-1167.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- Carter C, Braver T, Barch D, Botvinick M, Noll D, Cohen J. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chahboune H, Mishra A, DeSalvo M, Staib L, Purcaro M, Scheinost D, Papademetris X, Fyson S, Lorincz M, Crunelli V, Hyder F, Blumenfeld H. DTI abnormalities in anterior corpus callosum of rats with spike-wave epilepsy. Neuroimage. 2009;47:459–466. doi: 10.1016/j.neuroimage.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Briellmann R, Pell G, Scheffer I, Abbott D, Jackson G. Thalamic atrophy in childhood absence epilepsy. Epilepsia. 2006;47:399–405. doi: 10.1111/j.1528-1167.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- Conners C, Epstein J, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. J Abnorm Child Psychol. 2003;3:555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade J, Ollinger J, McAvoy M, Shulman G. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman G. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Tansy A, Stanley C, Astafiev S, Snyder A, Shulman G. A functional MRI study of preparatory signals for spatial location and objects. Neuropsychologia. 2005;43:2041–2056. doi: 10.1016/j.neuropsychologia.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Dosenbach N, Fair D, Miezin F, Cohen A, Wenger K, Dosenbach R, Fox M, Snyder A, Vincent J, Raichle M, Schlaggar B, Petersen S. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N, Nardos B, Cohen A, Fair D, Power J, Church J, Nelson S, Wig G, Vogel A, Lessov-Schlaggar C, Barnes K, Dubis J, Feczko E, Coalson R, Pruett J, Jr, Barch D, Petersen S, Schlaggar B. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N, Visscher K, Palmer E, Miezin F, Wenger K, Kang H, Burgund E, Grimes A, Schlaggar B, Petersen S. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C, Mirsky A, Lovelace C, WH WT. Assessment of the attention impairment in absence epilepsy: comparison of visual and auditory P300. Int J Pshychopysiol. 2009;73:118–122. doi: 10.1016/j.ijpsycho.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn D, Austin J, Harezlak J, Ambrosius W. ADHD and epilepsy in childhood. Dev Med Child Neurol. 2003;45:50–54. [PubMed] [Google Scholar]
- Eckert M, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno J. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30:2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JJ, Lubens P, Kuhl D, Phelps M. Local cerebral metabolic rate for glucose during petit mal absences. Ann Neurol. 1985;17:121–128. doi: 10.1002/ana.410170204. [DOI] [PubMed] [Google Scholar]
- Epstein J, Erkanli A, Conners C, Klaric J, Costello J, Angold A. Relations between Continuous Performance Test performance measures and ADHD behaviors. J Abnorm Child Psychol. 2003;31:543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- Fair D, Cohen A, Power J, Dosenbach N, Church J, Miezin F, Schlaggar B, Petersen S. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D, Dosenbach N, Church J, Cohen A, Brahmbhatt S, Miezin F, Barch D, Raichle M, Petersen S, Schlaggar B. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss B, Fossella J, Flombaum J, Posner M. The activation of attentional networks. Neuroimage. 2005a;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005b;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, Posner M. Human attentional networks. Psychiatr Prax. 2004;31:S210–214. doi: 10.1055/s-2004-828484. [DOI] [PubMed] [Google Scholar]
- Fedio P, Mirsky A. Selective intellectual deficits in children with temporal lobe or centrencephalic epilepsy. Neuropsychologia. 1969;7:287–300. [Google Scholar]
- Fojtiková D, Brázdil M, Horký J, Mikl M, Kuba R, Krupa P, Rektor I. Magnetic resonance spectroscopy of the thalamus in patients with typical absence epilepsy. Seizure. 2006;15:533–540. doi: 10.1016/j.seizure.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Fox M, Corbetta M, Snyder A, Vincent J, Raichle M. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Holmes A, Poline J, Price C, Frith C. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Gehring W, Knight R. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Giraud A, Kell C, Thierfelder C, Sterzer P, Russ M, Preibisch C, Kleinschmidt A. Contributions of sensory input, auditory search and verbal comprehension to cortical activity during speech processing. Cereb Cortex. 2004;14:247–255. doi: 10.1093/cercor/bhg124. [DOI] [PubMed] [Google Scholar]
- Glauser T, Cnaan A, Shinnar S, Hirtz D, Dlugos D, Masur D, Clark P, Capparelli E, Adamson P, Group CAES. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362:790–799. doi: 10.1056/NEJMoa0902014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci U S A. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamandi K, Salek-Haddadi A, Laufs H, Liston A, Friston K, Fish D, Duncan J, LLL EEG-fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage. 2006;31:1700–1710. doi: 10.1016/j.neuroimage.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth J, Gore J, RT, RC Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Neuroimage. 2010 Apr;:20. doi: 10.1016/j.mri.2010.03.021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer R, Skudlarski P, Gore J, Constable R. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. Neuroimage. 2006;31:513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Henkin Y, Sadeh M, Kivity S, Shabtai E, Kishon-Rabin L, Gadoth N. Cognitive function in idiopathic generalized epilepsy of childhood. Dev Med Child Neurol. 2005;47:126–132. doi: 10.1017/s0012162205000228. [DOI] [PubMed] [Google Scholar]
- Holdsworth L, Whitmore K. A study of children with epilepsy attending ordinary schools. Dev Med Child Neurol. 1974;16:746–758. doi: 10.1111/j.1469-8749.1974.tb03395.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. Yale University Press; New Haven, CT: 1976. [Google Scholar]
- Holmes A, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- Hopfinger J, Buonocore M, Mangun G. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Iannetti P, Spalice A, Luca PD, Boemi S, Festa A, Maini C. Ictal single photon emission computed tomography in absence seizures: apparent implication of different neuronal mechanisms. J Child Neurol. 2001;16:257–263. doi: 10.1177/088307380101600506. [DOI] [PubMed] [Google Scholar]
- ILAE. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Coordination Active du Réseau Observatoire Longitudinal de l’ Epilepsie. Epilepsia. 2001;42:464–475. doi: 10.1046/j.1528-1157.2001.31400.x. [DOI] [PubMed] [Google Scholar]
- Jenkins G, Watts D. Spectral analysis and its applications. Holden-Day; San Francisco, CA: 1968. [Google Scholar]
- Jus A, Jus C. Etude electro-clinique des alterations de conscience dans le petit mal. Studii si cercetari de. Neurol. 1960;5:243–254. [Google Scholar]
- Kapucu L, Serdaroğlu A, Okuyaz C, Köse G, Gücüyener K. Brain single photon emission computed tomographic evaluation of patients with childhood absence epilepsy. J Child Neurol. 2003;18:542–548. doi: 10.1177/08830738030180080401. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk M, Weerd PD, Desimone R, Ungerleider L. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kelly A, Di Martino A, Uddin L, Shehzad Z, Gee D, Reiss P, Margulies D, Castellanos F, Milham M. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kerns J, Cohen J, 3rd, AM, Cho R, Stenger V, Carter C. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kincade J, Abrams R, Astafiev S, Shulman G, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bossaerts P. Neural antecedents of financial decisions. J Neurosci. 2007;27:8174–8177. doi: 10.1523/JNEUROSCI.1564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer G, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labate A, Briellmann R, Abbott D, Waites A, Jackson G. Typical childhood absence seizures are associated with thalamic activatio. Epileptic Disord. 2005;7:373–377. [PubMed] [Google Scholar]
- Laufs H, Lengler U, Hamandi K, Kleinschmidt A, Krakow K. Linking generalized spike-and-wave discharges and resting state brain activity by using EEG/fMRI in a patient with absence seizures. Epilepsia. 2006;47:444–448. doi: 10.1111/j.1528-1167.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- Levav M, Mirsky A, Herault J, Xiong L, Amir N, Andermann E. Familial association of neuropsychological traits in patients with generalized and partial seizure disorders. J Clin Exp Neuropsychol. 2002;24:311–326. doi: 10.1076/jcen.24.3.311.985. [DOI] [PubMed] [Google Scholar]
- Li Q, Luo C, Yang T, Yao Z, He L, Liu L, Xu H, Gong Q, Yao D, Zhou D. EEG-fMRI study on the interictal and ictal generalized spike-wave discharges in patients with childhood absence epilepsy. Epilepsy Res. 2009;87:160–168. doi: 10.1016/j.eplepsyres.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, Luo C, Lu G, Chen H. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. 2010;5:e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Powell D, Wang H, Gold B, Corbly C, Joseph J. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Ouyang L, Chen Q, Huang X, Tang H, Chen H, Zhou D, Kemp G, Gong Q. Differential interictal activity of the precuneus/posterior cingulate cortex revealed by resting state functional MRI at 3T in generalized vs. partial seizure. J Magn Reson Imaging. 2008;27:1214–1220. doi: 10.1002/jmri.21370. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker D. Medial frontal cortex in action monitoring. J Neurosci. 2000;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren H, Pijn J, van Luijtelaar E, Coenen A, Lopes da Silva F. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol. 2005;62:371–376. doi: 10.1001/archneur.62.3.371. [DOI] [PubMed] [Google Scholar]
- Mirsky A, Buren JV. On the nature of the “absence” in centrencephalic epilepsy: A study of some behavioral, electroencephalographic and autonomic factors. Electroencephalogr Clin Neurophysiol. 1965;18:334–348. doi: 10.1016/0013-4694(65)90053-2. [DOI] [PubMed] [Google Scholar]
- Mirsky M, Primaca D, Marsana C, Rosvolda H, Stevens J. A comparison of the psychological test performance of patients with focal and nonfocal epilepsy. Experimental Neurology. 1960;2:75–89. doi: 10.1016/0014-4886(60)90049-2. [DOI] [PubMed] [Google Scholar]
- Mitchell W, Zhou Y, Chavez J, Guzman B. Reaction time, attention, and impulsivity in epilepsy. Pediatr Neurol. 1992;8:19–24. doi: 10.1016/0887-8994(92)90047-3. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner H, Wolff S, Muhle H, Boor R, Granert O, Jansen O, Stephani U, Siniatchkin M. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. Neuroimage. 2008a;39:1839–1849. doi: 10.1016/j.neuroimage.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner H, Wolff S, Muhle H, Granert O, Jansen O, Stephani U, Siniatchkin M. Simultaneous EEG-fMRI in drug-naive children with newly diagnosed absence epilepsy. Epilepsia. 2008b;49:1510–1519. doi: 10.1111/j.1528-1167.2008.01626.x. [DOI] [PubMed] [Google Scholar]
- Morgan V, Gore J, Abou-Khalil B. Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy Res. 2010;88:168–178. doi: 10.1016/j.eplepsyres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi M, Abildgaard M, Nixon T, RT RC. Removal of time-varying gradient artifacts from EEG data acquired during continuous fMRI. Clin Neurophysiol. 2004;115:2181–2192. doi: 10.1016/j.clinph.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Negishi N, Abildgaard M, Laufer I, Nixon T, Constable R. An EEG (electroencephalogram) recording system with carbon wire electrodes for simultaneous EEG-fMRI (functional magnetic resonance imaging) recording. J Neurosci Methods. 2008;173:99–107. doi: 10.1016/j.jneumeth.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersesyan H, Hyder F, Rothman D, Blumenfeld H. Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. 2004;24:589–599. doi: 10.1097/01.WCB.0000117688.98763.23. [DOI] [PubMed] [Google Scholar]
- Nomura E, Gratton C, Visser R, Kayser A, Perez F, D’Esposito M. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci USA. 2010;107:12017–12022. doi: 10.1073/pnas.1002431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I, Campenhausen G. Social adjustment in young adults with absence epilepsies. 1993;34:846–851. doi: 10.1111/j.1528-1157.1993.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Posner M, Petersen S, Fox P, Raichle M. Localization of cognitive operations in the human brain. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, LLL, Merschhemke M, Friston K, Duncan J, Fish D. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003;53:663–667. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- Seeley W, Menon V, Schatzberg A, Keller J, Glover G, Kenna H, Reiss A, Greicius M. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman E, Slick D, Connolly M, Eyrl K. ADHD, neurological correlates and health-related quality of life in severe pediatric epilepsy. Epilepsia. 2007;48:1083–1091. doi: 10.1111/j.1528-1167.2007.01028.x. [DOI] [PubMed] [Google Scholar]
- Shulman G, McAvoy M, Cowan M, Astafiev S, Tansy A, d’Avossa G, Corbetta M. Quantitative analysis of attention and detection signals during visual search. J Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Shulman G, Pope D, Astafiev S, McAvoy M, Snyder A, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010;30:3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter H, Giesbrecht B, Kok A, Weissman D, Kenemans J, Woldorff M, Mangun G. fMRI evidence for both generalized and specialized components of attentional control. Brain Res. 2007;1177:90–102. doi: 10.1016/j.brainres.2007.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin D, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tana M, Montin E, Cerutti S, Bianchi A. Exploring cortical attentional system by using fMRI during a Continuous Perfomance Test. Comput Intell Neurosci. 2010;329213:6. doi: 10.1155/2010/329213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore W, Brooks R, Margolin R, Patronas N, Sato S, Porter R, Mansi L, Bairamian D, DiChiro G. Positron emission tomography in generalized seizures. Neurology. 1985;35:684–690. doi: 10.1212/wnl.35.5.684. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Pessoa L. Neural correlates of perceptual choice and decision making during fear-disgust discrimination. J Neurosci. 2007;27:2908–2917. doi: 10.1523/JNEUROSCI.3024-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu P, Buckner R, Zollei L, Dyckman K, Goff D, DSDM Reduced functional connectivity in a right-hemisphere network for volitional ocular motor control in schizophrenia. Brain. 2010;133:625–637. doi: 10.1093/brain/awp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C, Vestal M, DeSalvo M, Berman R, Chung M, Blumenfeld H, Spann M. Differentiation of attention-related problems in childhood absence epilepsy. Epilepsy & Behavior. 2010;19:82–85. doi: 10.1016/j.yebeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites A, Briellmann R, Saling M, Abbott D, Jackson G. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59:335–343. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O’Keefe K, Putcha D, Dijk ABKV, Pihlajamäki M, Dickerson B, Sperling R. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51:910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. 1. Pearson; San Antonio, TX: 1999. [Google Scholar]
- Wickens TD. Elementary Signal Detection Theory. Oxford University Press, Inc; New York, NY: 2002. [Google Scholar]
- Williams J, Lange B, Phillips T, Sharp G, DelosReyes E, Bates S, Griebel M, Simpson P. The course of inattentive and hyperactive-impulsive symptoms in children with new onset seizures. Epilepsy Behav. 2002;3:517–521. doi: 10.1016/s1525-5050(02)00532-2. [DOI] [PubMed] [Google Scholar]
- Wirrell E, Camfield C, Camfield P, Dooley J, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy. Sometimes a wolf in sheeps’ clothing. Arch Pediatr Adolesc Med. 1997;151:152–158. doi: 10.1001/archpedi.1997.02170390042008. [DOI] [PubMed] [Google Scholar]
- Woods R, Grafton S, Holmes C, Cherry S, Mazziotta J. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Yeager C, Guerrant J. Subclinical epileptic seizures; impairment of motor performance and derivative difficulties. Calif Med. 1957;86:242–247. [PMC free article] [PubMed] [Google Scholar]
- Yeni S, Kabasakal L, Yalçinkaya C, Nişli C, Dervent A. Ictal and interictal SPECT findings in childhood absence epilepsy. Seizure. 2000;9:265–269. doi: 10.1053/seiz.2000.0400. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Chen Z, Shi J, Liu Y. Impaired perceptual networks in temporal lobe epilepsy revealed by resting fMRI. J Neurol. 2009a;256:1705–1713. doi: 10.1007/s00415-009-5187-2. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Yang Z, Liao W, Chen Z, Shi J, Liu Y. Impaired attention network in temporal lobe epilepsy: a resting FMRI study. Neurosci Lett. 2009b;458:97–101. doi: 10.1016/j.neulet.2009.04.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BOLD fMRI changes during the RTT task. A. t map demonstrating increases (hot colors) and decreases (cold colors) during RTT (N = 47; 25 patients, 22 controls) relative to fixation (height threshold p<0.05, FWE corrected, extent threshold k = 3 voxels). Significant fMRI increases included the upper extremity portion of the left motor cortex, the supplementary motor area, the right anterior insula, right frontal cortex, and right parietal cortex.
Correlation analyses between CAE patients’ attentional performance and mean beta values within the medial frontal ROI during CPT. Note that decreased medial frontal cortex (MFC) activation was correlated with all measures of worse CPT performance, including increased reaction time (A), increased standard deviation of reaction time (B), and decreased d-prime (C). Data are for same patients as shown for omission error rate in Figure 1C (n = 26).
Resting functional connectivity analysis for right and left In/FO seed regions. A. Left anterior insula/frontal operculum ROI seed region shows maximal connectivity with medial frontal cortex and right anterior insula/frontal operculum, B. Right anterior insula/frontal operculum ROI seed region shows maximal connectivity with medial frontal cortex and left anterior insula/frontal operculum, Note that findings parallel the attention network activated during the CPT task. t maps with n = 32 (16 patients, 16 controls), height threshold was made more stringent at p<0.001, FWE corrected to reveal connectivity maxima, extent threshold k = 3 voxels. Same data and subjects as in Figure 2.
Demographics of the RTT cohort and connectivity (fixation) cohort.