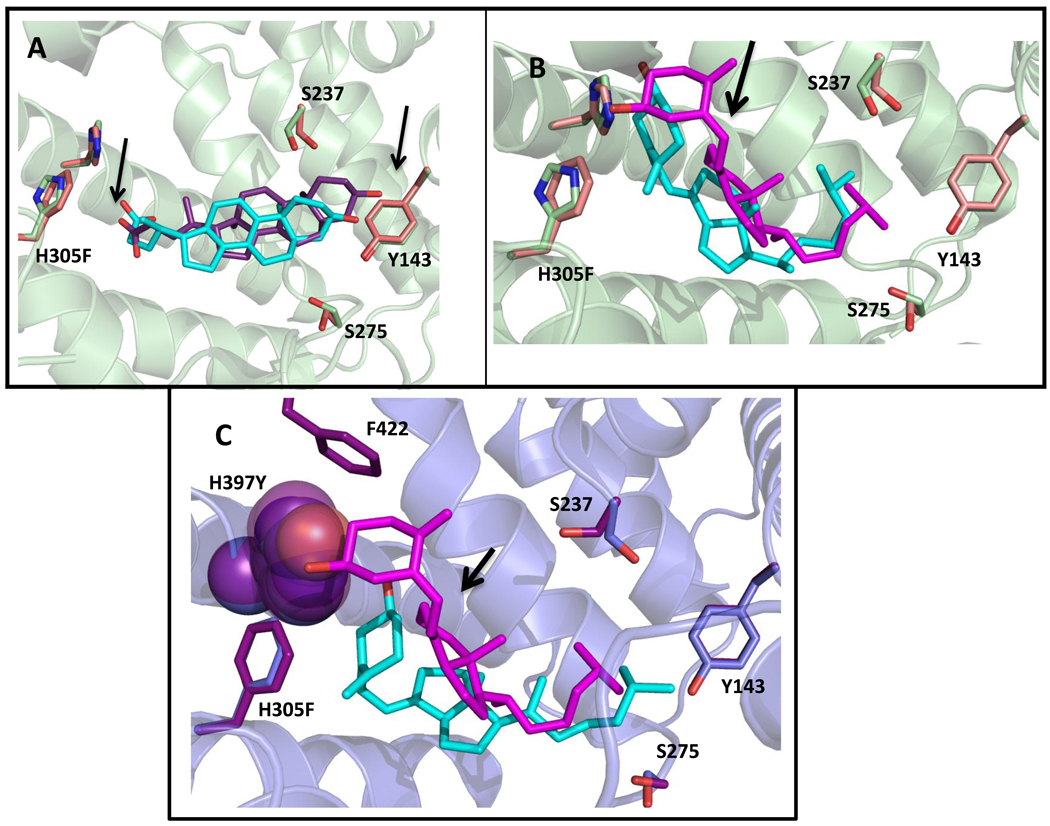
Figure 6. Results of docking lithocholic acid and cholecalciferol to wild-type hVDR and variants.
Wild-type docked structures are green (residues) and cyan (ligand). Variant docked structures are pink/blue (residues) and purple/pink (ligand). In A, as shown with the arrows, an upward shift in the pocket and a change in position of the carboxyl of LCA, places the carboxyl group into closer proximity to the phenylalanine, which may be responsible for the increased activity of H305F with LCA. In B, cholecalciferol is flipped 180° from the conformation seen for 1,25(OH)2D3 in the wild-type crystal structure (Figure 3). This flipped conformation is also observed when 3-keto lithocholic acid is docked to hVDR [22]. The arrow shows a slight shift of the cholecalciferol bound to H305F (pink) when compared to wild-type (cyan). C shows a spacefill of H397 (blue) and H397Y (purple) in order to show the difference in volumes leading to a drastic shift of the cholecalciferol to a more bowl-shaped conformation in the pocket in H305F/H397Y (cyan) in comparison to H305F (pink). The distance between the hydroxyl group of cholecalciferol and the hydrogen bonding atom on residue 397 (H and Y) is annotated. The binding energies are as follows: wild-type hVDR/LCA (−9.7 kcal/mol), H305F/LCA (−9.6 kcal/mol), wild-type hVDR/cholecalciferol (−10.4 kcal/mol), H305F/cholecalciferol (−10.6 kcal/mol), and H305F/H397Y/cholecalciferol (−10.2 kcal/mol).