Abstract
Postmortem studies have documented abnormalities in the dorsolateral prefrontal cortex (dlPFC) in depressed subjects. In this study we used magnetic resonance imaging to test for dlPFC volume differences between older depressed and non-depressed individuals. Eighty-eight subjects meeting DSM IV criteria for major depressive disorder and thirty-five control subjects completed clinical evaluations and cranial 3T magnetic resonance imaging. After tissue types were identified using an automated segmentation process, the dlPFC was measured in both hemispheres using manual delineation based on anatomical landmarks. Depressed subjects had significantly lower gray matter in left and right dorsolateral prefrontal cortex (standardized to cerebral parenchyma) after controlling for age and sex. Our study confirmed the reduction of dorsolateral prefrontal cortex in elderly depressed subjects, especially in the gray matter. These regional abnormalities may be associated with psychopathological changes in late-life depression.
Keywords: magnetic resonance imaging, elderly, mood
1. Introduction
Several studies have examined the effect of aging on prefrontal brain volumes in healthy subjects (Raz et al., 1997; Raz et al., 2004; Walhovd et al., 2005) but few studies have focused on the volumetric differences of dorsolateral prefrontal cortex (dlPFC) in geriatric depression. The dlPFC, comprised of the superior and middle frontal gyri (Crespo-Facorro et al., 2000), is an important region of the prefrontal cortex which receives projections from higher order association regions (Nauta, 1971). It projects to the dorsolateral head of the caudate nucleus, continues to the lateral dorsomedial globus pallidus and, finally, to the ventral anterior and mediodorsal thalamus (Tekin and Cummings, 2002). Previous studies have shown that the dlPFC has extensive connectivity to cortical and subcortical circuits that may underlie its importance in modulating mood regulation and cognitive function (Duffy and Campbell, 1994). Prior research has shown that abnormalities in the dlPFC are implicated in the pathology of late-life depression (Thomas et al., 2000; Thomas et al., 2003; Taylor et al., 2004). However, a recent meta-analysis examining functional magnetic resonance imaging (MRI) studies of depression revealed no consistent pattern of abnormalities in dlPFC activity, a result that may be due to methodologic variability, clinical heterogeneity of the depressive subjects or different imaging paradigms (Fitzgerald et al., 2006).
Most anatomic studies of the dlPFC in depression have been performed postmortem. Studies have reported marked reduction in the density and size of neurons and glial cells in both supra- and infragranular layers (Rajkowska et al., 1999) as well as a decrease in pyramidal neuronal size in the overall cortex (Khundakar et al., 2009). Thomas et al. have shown that, compared with control subjects, depressed individuals exhibit higher ischemia-induced inflammation in gray and white matter of the dlPFC (Thomas et al., 2000; Thomas et al., 2002), providing evidence for the “vascular depression” hypothesis linking cerebrovascular disease with late-life depression (Alexopoulos et al., 1997; Krishnan et al., 1997). In younger adult populations, brain imaging studies have reported that unipolar depressed subjects, compared with healthy controls, have clusters of decreased gray matter density (T. Frodl et al., 2010) as well as decreased dlPFC glucose metabolism and blood flow (Ketter et al., 1996; Drevets, 1998). Antidepressant treatment appears to increase metabolic activity in the middle frontal gyrus in depressive subjects (Buchsbaum et al., 1997) and to normalize metabolism in the prefrontal cortex (Brody et al., 2001) while remission is associated with less decline in dlPFC gray matter (T. S. Frodl et al., 2008). Reduced dlPFC activity has been linked to severity of illness (Drevets, 1998) and to cognitive disturbance (Bench et al., 1992; Bench et al., 1993). However, there is limited in vivo evidence and no large-sample studies of dlPFC volume in late-life depression.
In this study we evaluated dlPFC volume in subjects with late-life depression compared with a group of never-depressed elderly control subjects. We hypothesized that older subjects with depression would have smaller dlPFC volumes than older comparison subjects.
2. Methods
2.1. Participants
This cross-sectional project occurred within a larger longitudinal clinical study of depression in older adults, the Conte Center for the Neuroscience of Depression and Neurocognitive Outcomes of Depression in the Elderly (NCODE) study. Eighty-eight depressed subjects and 35 control subjects were examined in this study. The study was approved by Duke University Medical Center’s Institutional Review Board. All enrolled subjects were 60 years or older. Depressed subjects were recruited from the Duke University Medical Center psychiatric inpatient and outpatient services and met criteria for DSM-IV major depressive episode. Age of onset of current episode was not limited to a specific age nor was there a requirement that subjects have no prior episodes of depression. Exclusion criteria included 1) another major psychiatric illness, such as bipolar disorder, schizophrenia, and schizoaffective disorder; 2) history of alcohol or drug abuse or dependence; 3) primary neurologic illness, such as dementia, stroke, Parkinson disease, seizure disorder, and multiple sclerosis; 4) medications, medical illness, or physical disability that may severely affect cognitive function and ability to provide consent; 5) physical disability which precludes cognitive testing; and 6) metal in the body which precludes MRI. Comparison subjects were required to have a non-focal neurological examination, self-report of no current or past neurological or depressive illness, and no evidence of a depression diagnosis based on the Diagnostic Interview Schedule portion of the Duke Depression Evaluation Schedule.
Subjects were assessed with a Mini-Mental State Examination (MMSE) (Folstein et al., 1975) at baseline. All control subjects had MMSE scores of 25 or higher. If a depressed subject had an MMSE score less than 25, subjects were followed through an acute (eight-week) phase of treatment to determine if cognition improved. Subjects whose MMSE scores remained below 25 were not followed longitudinally. Thus, in the clinical judgment of the study geriatric psychiatrist and by established NCODE protocol, dementia was effectively excluded at or close to baseline in all elderly depressed NCODE subjects.
2.2. Depression treatment
Depressed participants received individualized treatment from a psychiatrist, who followed them throughout the study. Most received antidepressant medication; some received electroconvulsive treatment (ECT) or psychotherapy.
2.3. MRI scanning protocol
Cranial MRI was performed using the 8-channel parallel imaging head coil on a 3 Tesla whole-body MRI system (Trio, Siemens Medical Systems, Malvern, PA). Proton density (PD), T1-weighted, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) images were acquired. Parallel imaging was employed with an acceleration factor of 2.
The T1-weighted image set was acquired using a 3D axial TURBOFLASH sequence with TR/TE=22/7 msec, flip angle=25°, a 100 Hz/pixel bandwidth, a 256×256 matrix, a 256 mm diameter field-of-view, 160 slices with a 1 mm slice thickness and Nex=1 (no signal averaging), yielding an image with 1 mm cubic voxels in an 8 minute, 18 second imaging time. This was followed by a T2-weighted acquisition using a 2D turbo spin-echo pulse sequence with TR/TE=7580/86 msec, turbo factor=7, a 210 Hz/pixel bandwidth, a 256×256 matrix, a 256 mm diameter field-of-view, 100 slices with a 1.5 mm slice thickness and Nex=1 (no signal averaging), yielding a 1×1×1.5 mm voxel in a 5 minute, 21 second imaging time. Next a PD weighted volume was acquired with a 2D turbo spin-echo pulse sequence with TR/TE=7580/17 msec, turbo factor=3, a 210 Hz/pixel bandwidth, a 256×256 matrix, a 256 mm diameter field-of-view, 100 slices with a 1.5 mm slice thickness and Nex=1 (no signal averaging), yielding a 1×1×1.5 mm voxel in a 6 minute, 21 second imaging time. Finally, a FLAIR image was acquired with TR/TI/TE=9000/2400/101 msec, a 210 Hz/pixel bandwidth, a turbo factor of 11, a 256×256 matrix, a 256 mm diameter field-of-view, 75 slices with a 2 mm slice thickness and Nex=1 (no signal averaging), yielding a 1×1×2 mm voxel in a 9 minute, 45 second imaging time.
2.4. Whole brain segmentation
The MR images were transferred to the Duke Neuropsychiatric Imaging Research Laboratory (NIRL) where all image analyses were performed. Images were initially resliced to a common geometry of 1×1×1.5mm voxels. An automated 4-channel lesion segmentation, which takes advantage of FLAIR images for lesion detection, was performed to assess gray matter, white matter, cerebrospinal fluid, and white matter lesions. The algorithm used was a variation on the fully automated Expectation Maximization Segmentation (EMS) method (Van Leemput et al., 1999; Van Leemput et al., 2001; Van Leemput et al., 2003). The method was optimized for vascular lesion assessment in elderly subjects. The software assigns a probability that a given pixel should be classified as gray matter, white matter, cerebrospinal fluid, lesion or non-brain in the following manner. First, images are aligned to a set of tissue probability images using the mutual information registration tool (MIRIT) (Maes et al., 1997). The probability atlas provides spatial priors for each tissue that are used to initialize the tissue intensity histograms for the segmentation algorithm. The tissue probabilities are then derived in an iterative process using the intensity distributions of the different tissues for each of the input image contrasts. The process also evaluates and compensates for spatial distributions of intensity that could be due to various magnetic resonance imaging artifacts such as radiofrequency inhomogeneity (bias correction). Lesions are detected as ‘outliers’ to the normal tissue distributions. This method requires parameter optimization for each dataset due to variations in subject populations and scanners. After identifying the optimal parameters for a dataset, the method is fully automated. This automation provides an advantage over semi-automated methods which require an analyst to choose seeding points and lesion regions. The method is capable of distinguishing and classifying lesions and other brain tissues simultaneously.
2.5. Volumetry of dlPFC
For this project, the dlPFC was defined as consisting of Superior Frontal Gyrus (SFG) and Middle Frontal Gyrus (MFG).The tracing procedures were modified from Crespo-Facorro et al. (Crespo-Facorro et al., 2000). Tracing was performed with the ITK-SNAP 1.4.1 program (Yushkevich et al., 2006). The dlPFC tracing mask was applied to the automated segmentation in order to obtain volumes for left and right hemisphere gray and white matter in the dlPFC.
Superior Cingulate Sulcus (SCiS) and Superior Rostral Sulcus (SRS) were defined as the medio-inferior border, Inferior Frontal Sulcus (IFS) as the latero-inferior border, Central Sulcus (CS) as the medial border, and PreCentral Sulcus (PCS) as the posterior border. See Table 3 for full list of abbreviations. Tracing was separated into 4 steps, described below.
Table 3.
Abbreviations
| Abbreviation | Anatomical Definition |
|---|---|
| CS | Central Sulcus |
| Cis | Cingulate Sulcus |
| dlPFC | Dorsolateral Prefrontal Cortex |
| FMS | FrontoMarginal Sulcus |
| IFG | Inferior Frontal Gyrus |
| IFS | Inferior Frontal Sulcus |
| MFG | Middle Frontal Gyrus |
| PCS | Pre-Central Sulcus |
| SciS | Superior Cingulate Sulcus |
| SFG | Superior Frontal Gyrus |
| SFS | Superior Frontal Sulcus |
| SRS | Superior Rostral Sulcus |
-
Step 1
Trace SFG in coronal view (Fig. 1). SFG tracing began on the coronal slice identified as Plane A. Plane A (Fig. 2) is a coronal plane passing through the anterior extent of the inner surface of the genu (corpus callosum). Plane A serves as the posterior boundary for coronal tracing of SFG. The superior frontal sulcus (SFS) was followed superiorly, tracing around brain cortex medially to the midline, then inferio-laterally into superior cingulate sulcus (SCiS). The sagittal view was used to identify the SCiS as the medial-inferior boundary of SFG. While tracing SFG anteriorly, tracing continued superiorly to the SCiS or Cingulate Sulcus (CiS) and excluded superior cingulate gyrus/cingulate gyrus. When the SCis/CiS descended inferiorly, there was a transition to the SRS as the new medial-inferior boundary of SFG. Tracing continued anteriorly until the end of the SRS.
-
Step 2
Trace SFG in axial view. To include SFG posterior to Plane A slice, tracing was done in the axial view. Plane A serves as the anterior boundary for axial tracing of SFG. The SFS is the lateral boundary of SFG. On an axial slice with the most posterior point of SFS, a straight, horizontal line was drawn to midline CSF, designating the posterior boundary. The midline is the medial boundary and plane A is the anterior boundary. Tracing continued slice by slice superiorly to include superior portions of SFG. Once the superior-most extent of SFG was reached, tracing continued inferiorly from the starting axial slice using the same guidelines as above. Once SFS retreated anteriorly beyond Plane A, SFG tracing was stopped.
-
Step 3
Trace MFG in coronal view (Fig. 3). Plane A serves as the posterior boundary for coronal tracing of MFG. In the sagittal view, the IFS was identified on a slice where the Sylvian fissure is clearly presented. The IFS, which serves as the lateral and inferior boundary of MFG, is the most superior sulcus parallel (anterior-posterior orientation) to the Sylvian Fissure. Trace was done laterally in the coronal view from the IFS then superiorly around MFG to the deepest SFS point, where a straight line was drawn connecting the deepest SFS point to the deepest IFS point. Tracing continued anteriorly on each slice until the IFS branch/FrontoMarginal Sulcus (FMS) disappeared.
-
Step 4
Trace MFG in axial view. MFG tracing was completed in the axial view starting on the slice on which, due to the coronal tracing, MFG appears lateral to SFG. Plane A serves as the anterior boundary for axial tracing of MFG. Tracing began from the deepest point of the PCS, continued laterally and anteriorly around MFG to the deepest point of SFS, and then back to the PCS. Tracing of each axial slice was reviewed on the sagittal view to confirm exclusion of IFG. Tracing was discontinued when one of the following conditions was met: 1) all cortex above IFS on sagittal view was included or 2) IFG connected to MFG.
Figure 1.
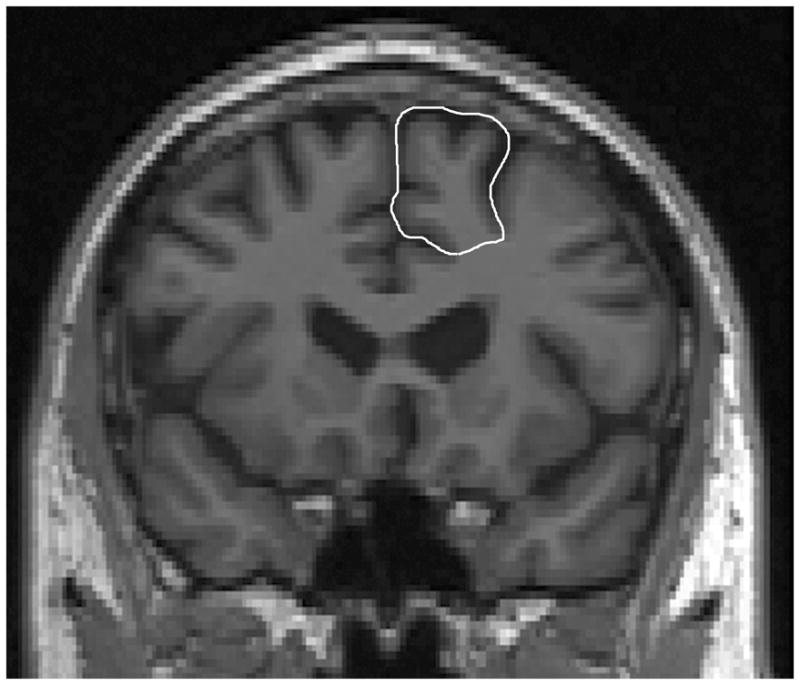
Superior frontal gyrus (SFG) tracing in coronal view.
Figure 2.
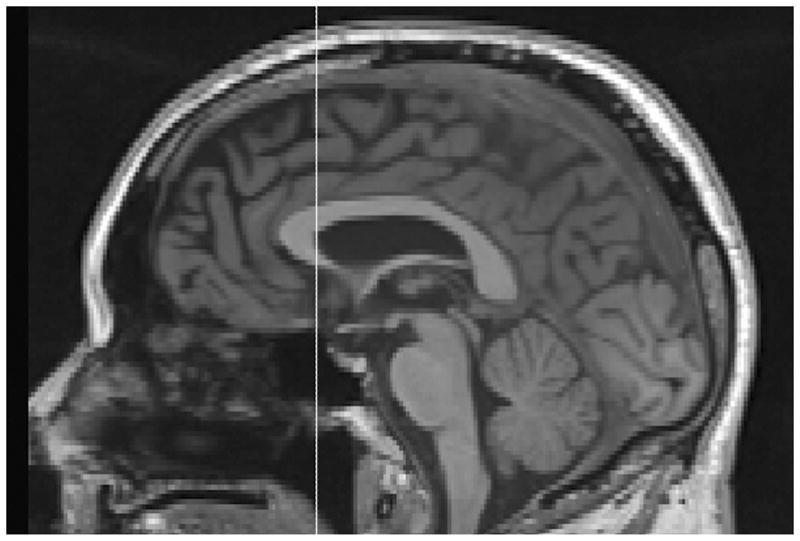
Plane A is anterior extent of inner surface of genu (corpus callosum) in sagittal view.
Figure 3.
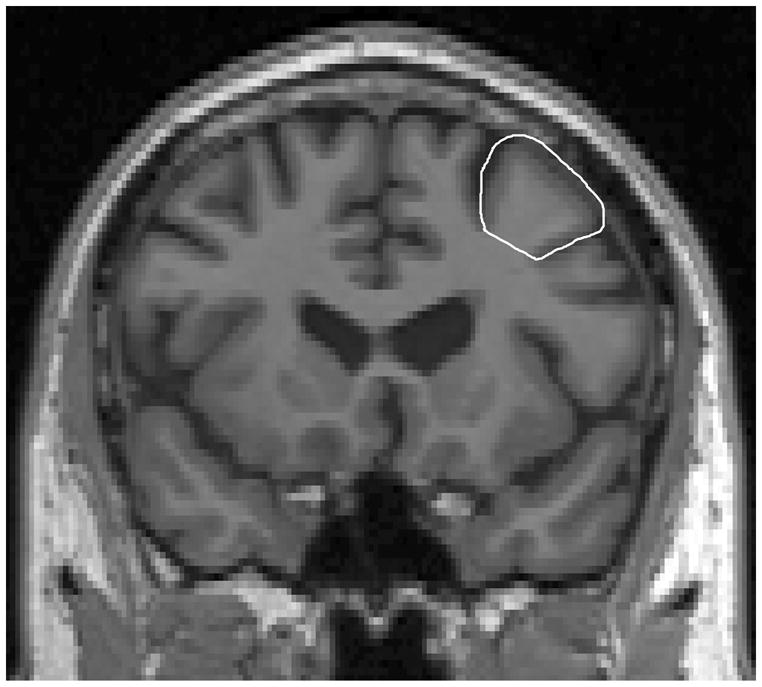
Middle frontal gyrus (MFG) tracing in coronal view.
2.5.1. Reliability
Reliability was established by repeat measurements on multiple MR scans (N=5) by two raters (SCY, DFM). Intraclass correlation coefficients (ICCs) for dlPFC were as follows: left gray matter = 0.90, left white matter = 0.97, right gray matter = 0.87, and right white matter = 0.98.
2.6. Statistical analyses
SAS version 9.2 (Cary, NC, USA) was used to analyze data. Initial comparisons testing for differences of raw means and frequencies using T-test and chi-square statistics were performed (data were described as mean [SD] or percentage [n]). Linear regression models were then used to compare dlPFC volumes standardized to cerebral parenchyma between depressed and control groups while controlling for age and sex.
3. Results
This study included 88 depressed subjects (28 males and 60 females) and 35 comparison subjects (11 males and 24 females). As shown in Table 1, the sex, race, and MMSE scores of the depressive and comparison subjects were not statistically different. However, depressed subjects were significantly younger than controls (t =4.58, P <0.0001). Age ranges were 60–84 years for depressives, and 62–90 years for comparison subjects.
Table 1.
Sociodemographic and imaging characteristicsa
| Variable | Patients (N = 88) | Controls (N = 35) | Test Statistic |
|---|---|---|---|
| mean (SD) | mean (SD) | ||
| Age | 69.1 (5.7) | 74.5 (6.5) | t = 4.58, df = 121, P <0.0001 |
| Sex | |||
| % Female (N) | 68.2 (60) | 68.6 (24) | chi-square = 0.0018, df = 1, P = 0.97 |
| Race | |||
| % Caucasian (N) | 83.0 (73) | 91.4 (32) | chi-square = 1.44, df = 1, P = 0.2302 |
| MMSE Scoreb | 28.6 (2.3) | 29.1(1.1) | t = 1.48, df =102, P = 0.14 |
| Total Parenchyma (cerebrum, ml) | 925.6 (100.1) | 941.1 (93.6) | t = 0.79, df = 121, P = 0.43 |
| Left dlPFC | |||
| Total Volume (ml) | 49.7 (8.5) | 52.0 (6.9) | t = 1.43, df = 121, P = 0.16 |
| Gray Matter (ml) | 28.7 (5.9) | 30.2 (4.8) | t = 1.33, df = 121, P = 0.19 |
| White Matter (ml) | 20.9 (5.2) | 21.7 (4.7) | t = 0.83, df = 121, P = 0.41 |
| Right dlPFC | |||
| Total Volume (ml) | 50.4 (10.1) | 52.0 (8.7) | t = 0.86, df = 121, P = 0.39 |
| Gray Matter (ml) | 28.8 (6.3) | 30.4 (5.4) | t = 1.35, df = 121, P = 0.18 |
| White Matter (ml) | 21.4 (6.0) | 21.5 (5.6) | t = 0.05, df = 121, P = 0.96 |
N=123
Mini-Mental State Examination; N=105
We examined the clinical characteristics of the depressed subjects. At enrollment, 91% of subjects reported recurrent depression (>1 episode during lifetime), with an average age of onset (initial episode) of 35.7 years (SD=20.7), and an average illness duration of 18.1 years (SD=29.3). At time of MRI, 92% of the depressed subjects had taken an antidepressant and 9% had received ECT (these data were unavailable for N=20 subjects); data were not collected on the number receiving psychotherapy.
In bivariate analyses, depressed subjects did not significantly differ from control subjects on total dlPFC volume, total brain parenchyma, or standardized dlPFC volumes (see Table 1). However, in multivariable models adjusting for age and sex, standardized gray matter volumes were significantly smaller in both left and right dlPFC (see Table 2).
Table 2.
Linear regression model of dorsolateral prefrontal cortex (dlPFC) volume in depressed subjects and non-depressed comparison subjects
| Region | Depressed group, N = 88 | Control group, N = 35 | F1,119 value | P value |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Standardized Left | ||||
| dlPFC1 | ||||
| Total Volume | 0.0530 (0.0067) | 0.0549 (0.0067) | 1.85 | 0.18 |
| Gray Matter | 0.0306 (0.0048) | 0.0329 (0.0048) | 5.87 | 0.0169 |
| White Matter | 0.0224 (0.0051) | 0.0220 (0.0051) | 0.23 | 0.63 |
| Standardized Right | ||||
| dlPFC1 | ||||
| Total Volume | 0.0538 (0.0088) | 0.0551 (0.0088) | 0.57 | 0.45 |
| Gray Matter | 0.0307 (0.0054) | 0.0332 (0.0054) | 5.06 | 0.0263 |
| White Matter | 0.0230 (0.0062) | 0.0219 (0.0062) | 0.75 | 0.39 |
Standardized volume is proportion to parenchyma (gray + white matter of cerebrum).
All models adjusted for age and sex.
Given the difference in age between depressed and comparison subjects, dlPFC models were run separately for each group. Age had a significant negative effect upon left gray matter for both depressed (F1,86=4.85, p=0.030) and comparison subjects (F1,33=7.86, p=0.008), but was significant for right gray matter only among comparison subjects (F1,33=10.56, p=0.003). Age was marginally significant for right gray matter in depressives (F1,86=2.90, p=0.09). Also, an age by depression group interaction term was evaluated and determined to be non-significant for all dlPFC variables.
Lastly, the dlPFC models were re-analyzed with the addition of MMSE score, to determine if cognitive status may have mediated the gray matter/depression relationship. Depression had a significant but lessened effect on left dlPFC gray matter (F1,100=2.02, p=0.0461) but was now marginally significant for right gray matter (F1,100=3.86, p=0.0523). In the left dlPFC model only, MMSE was significantly positively associated with gray matter volume (F1,100=2.59, p=0.0113).
4. Discussion
The principal finding of this study is that older depressed individuals exhibited smaller gray matter volumes in both the left and right dlPFC, after adjusting for age and sex. These associations may be partially mediated by cognitive status. As expected, age was negatively related to dlPFC gray matter volume; age-related decline did not appear to differ between groups. Our findings contribute to the growing evidence that the dlPFC is critically involved in depression. To our knowledge, this is the first in vivo magnetic resonance imaging study to demonstrate smaller dlPFC gray matter volumes in late-life depression.
Major depressive disorder is characterized by disruptions in executive control, linked to abnormal dlPFC function. The dlPFC plays an important role in working memory and other aspects of executive function (Braver et al., 1997; D'Esposito et al., 2000). Previous studies have focused on younger populations; in vivo morphologic change of dlPFC in late-life depression has not been investigated. Of the studies in younger subjects, one postmortem neuropathological report demonstrated structural changes in the prefrontal regions in major depressive disorder subjects, including decreases in cortical thickness and neural size, together with reductions in neural and glial density (Rajkowska et al., 1999). In the study by Thomas et al., ischemia in the white matter of dlPFC was found in subjects with late-life depression (Thomas et al., 2003) and lends support to the “vascular depression” hypothesis (Alexopoulos et al., 1997; Krishnan et al., 1997). However the role of gray matter was not examined.
Our findings are consistent with studies linking the dlPFC with depression. In our previous study, we speculated that microstructural changes in white matter of the dlPFC may result in disconnection of cortical and subcortical regions (Taylor et al., 2004), while another study found that gray matter shrinkage was also involved in depression (Vasic et al., 2008). Functional imaging studies have been employed to identify brain areas involved in depression. Results from these studies associate depression with abnormally high levels of ventromedial activity (Drevets et al., 1992; Biver et al., 1994; Greicius et al., 2007) but abnormally low levels of dlPFC activity (Biver et al., 1994; Galynker et al., 1998). Our study expanded those findings to a larger sample and found that the dlPFC is critically involved in elderly depression, particularly the gray matter. Moreover, this relationship is linked to global cognition as measured by the MMSE, which supports past work linking geriatric depression with cognitive deficits, specifically executive dysfunction.
Separate examination of gray and white matter represents a methodological improvement over examining only the whole volume of a given brain structure. This is particularly important given that gray and white matter are composed of different cell types. White matter mostly consists of a variety of glial cells that provide myelination, support, and maintenance of the neurochemical environment. It is also where axonal projections between nerve bodies pass. Gray matter includes glia, but also nerve cell bodies, which project to other brain regions. The changes we found are likely to be localized primarily in cell bodies (neurons or glia) in the dlPFC gray matter.
Limitations of this study include potential bias given that control subjects were significantly older than depressed subjects. However, this difference would serve to create a bias against our hypothesis. Multivariable models did control for age which should have at least partially adjusted for this bias. In addition, an age by group interaction was examined and found to be non-significant. Other factors not included in this analysis, such as age of onset, illness duration, ECT, and prior antidepressant use, may influence the morphology of dlPFC in depression. Regarding the possible effects of treatment (antidepressants and ECT) upon dlPFC volume, a number of factors prevented their evaluation in these analyses, including missing data, heterogeneity of treatments, modest sample size, and use of polypharmacy. In addition, and perhaps most critical, was a lack of data on medication history. Since the majority of subjects had long-term depression (average episode duration = 18.1 years), they were likely to have taken antidepressants prior to study baseline; this prior medication use would likely have had a greater influence on brain structure than would concurrent medications, if any relationship exists. Finally, use of the MMSE provides limited information, particularly as this tool does not allow for a thorough examination of executive function, which is linked to geriatric depression. These factors should be addressed in future studies.
Our finding adds to the growing evidence that the dlPFC contributes to the neuroanatomic circuit related to mood regulation and depression. This is supportive of the proposed neuroanatomical models of mood regulation that involve prominent fronto-subcortical circuits (prefrontal cortex, amygdala, thalamus and basal ganglia)(Drevets et al., 1992). In addition to this cross-sectional study, longitudinal research is needed to determine the relationship between these changes and the pathogenesis of depression as well as the mechanisms by which these changes could predispose to depression.
We have shown that geriatric depressed subjects have significantly smaller dlPFC gray matter volumes than normal controls, supporting a role for dlPFC in the pathophysiology of late-life depression.
Acknowledgments
The authors would like to thank the following individuals from the Duke University Neuropsychiatric Imaging Research Laboratory: Ms. Cynthia Key for image analysis support, and Mr. Christopher Glessner and Dr. Robert Rybczynski for editorial assistance. This project was funded by the National Institute of Mental Health (P50 MH60451, R01 MH54846, and K24 MH70027).
Footnotes
Preliminary data were presented at the American Association for Geriatric Psychiatry Annual Meeting 2009 (Honolulu, Hawaii).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. American Journal of Psychiatry. 1997;154(4):562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychological Medicine. 1993;23(3):579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia--focal abnormalities of cerebral blood flow in major depression. Psychological Medicine. 1992;22(3):607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Biver F, Goldman S, Delvenne V, Luxen A, De Maertelaer V, Hubain P, Mendlewicz J, Lotstra F. Frontal and parietal metabolic disturbances in unipolar depression. Biological Psychiatry. 1994;36(6):381–388. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Phelps ME, Huang SC, Wu HM, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR., Jr Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Archives of General Psychiatry. 2001;58(7):631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, Reynolds C. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biological Psychiatry. 1997;41(1):15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim J, Andreasen NC, Spinks R, O'Leary DS, Bockholt HJ, Harris G, Magnotta VA. Cerebral cortex: a topographic segmentation method using magnetic resonance imaging. Psychiatry Research. 2000;100(2):97–126. doi: 10.1016/s0925-4927(00)00072-x. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Experimental Brain Research. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annual Review of Medicine. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. Journal of Neuroscience. 1992;12(9):3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JD, Campbell JJ., 3rd The regional prefrontal syndromes: a theoretical and clinical overview. Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6(4):379–387. doi: 10.1176/jnp.6.4.379. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Research. 2006;148(1):33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frodl T, Reinhold E, Koutsouleris N, Donohoe G, Bondy B, Reiser M, Moller HJ, Meisenzahl EM. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. 2010;35(6):1383–1390. doi: 10.1038/npp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, Scupin I, Reiser M, Moller HJ, Meisenzahl EM. Depression-related variation in brain morphology over 3 years: effects of stress? Archives of General Psychiatry. 2008;65(10):1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- Galynker II, Cai J, Ongseng F, Finestone H, Dutta E, Serseni D. Hypofrontality and negative symptoms in major depressive disorder. Journal of Nuclear Medicine. 1998;39(4):608–612. [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketter TA, George MS, Kimbrell TA, Benson BE, Post RM. Functional brain imaging, limbic function, and affective disorders. Neuroscientist. 1996;2:55–65. [Google Scholar]
- Khundakar A, Morris C, Oakley A, McMeekin W, Thomas AJ. Morphometric analysis of neuronal and glial cell pathology in the dorsolateral prefrontal cortex in late-life depression. British Journal of Psychiatry. 2009;195(2):163–169. doi: 10.1192/bjp.bp.108.052688. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. American Journal of Psychiatry. 1997;154(4):497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Transactions on Medical Imaging. 1997;16(2):187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- Nauta WJ. The problem of the frontal lobe: a reinterpretation. Journal of Psychiatry Research. 1971;8(3):167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry. 1999;45(9):1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiology of Aging. 2004;25(3):377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. American Journal of Psychiatry. 2004;161(7):1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. Journal of Psychosomatic Research. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Ferrier IN, Kalaria RN, Davis S, O'Brien JT. Cell adhesion molecule expression in the dorsolateral prefrontal cortex and anterior cingulate cortex in major depression in the elderly. British Journal of Psychiatry. 2002;181:129–134. doi: 10.1017/s0007125000161847. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Ferrier IN, Kalaria RN, Woodward SA, Ballard C, Oakley A, Perry RH, O'Brien JT. Elevation in late-life depression of intercellular adhesion molecule-1 expression in the dorsolateral prefrontal cortex. American Journal of Psychiatry. 2000;157(10):1682–1684. doi: 10.1176/appi.ajp.157.10.1682. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Perry R, Kalaria RN, Oakley A, McMeekin W, O'Brien JT. Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. International Journal of Geriatric Psychiatry. 2003;18(1):7–13. doi: 10.1002/gps.720. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Maes F, Vandermeulen D, Colchester A, Suetens P. Automated segmentation of multiple sclerosis lesions by model outlier detection. IEEE Transactions on Medical Imaging. 2001;20(8):677–688. doi: 10.1109/42.938237. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Transactions on Medical Imaging. 1999;18(10):897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Maes F, Vandermeulen D, Suetens P. A unifying framework for partial volume segmentation of brain MR images. IEEE Transactions on Medical Imaging. 2003;22(1):105–119. doi: 10.1109/TMI.2002.806587. [DOI] [PubMed] [Google Scholar]
- Vasic N, Walter H, Hose A, Wolf RC. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. Journal of Affective Disorders. 2008;109(1–2):107–116. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26(9):1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275–1268. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]


