Abstract
Hemodialysis patients who live at high altitude use less exogenous erythropoietin but achieve higher hematocrit levels than those living at a lower altitude. The authors hypothesized that the effect of altitude would be strongest in hemodialysis patients with poor anemia treatment response. To explore this hypothesis, they studied anemia-related outcomes in US hemodialysis patients who move to higher altitudes. Using Medicare and US Geological Survey data, in 1992–2004 they identified instances in which a patient moved from a dialysis center at an altitude of <2,000 feet (600 m) to one at a higher elevation. Of these moves, 5,274 were ≥3,000 feet (900 m; the altitude group) and 25,345 were 250–500 feet (75–150 m; the control group). Among patients with poor treatment response at baseline, large increases in hematocrit and decreases in erythropoietin dosing were observed in the altitude relative to the control group. At 6 months, hematocrit had increased more in the altitude group (5.1%, 95% confidence interval (CI): 4.1, 6.2 vs. 3.7%, 95% CI: 3.5, 3.9), and erythropoietin dosing decreased more (4,600 units/week, 95% CI: 500, 8,700 vs. 1,700 units/week, 95% CI: 1,000, 2,400). No effect of altitude was observed in patients with better treatment response at baseline. These results support the hypothesis that altitude-induced hypoxia reduces erythropoietin requirements in hemodialysis patients with treatment-refractory anemia.
Keywords: altitude; anemia; erythropoietin; kidney failure, chronic; risk factors
Patients with chronic kidney disease, including those requiring chronic hemodialysis, produce insufficient amounts of endogenous erythropoietin to maintain normal hematocrit levels. Replacement of lost erythropoietin production by administering recombinant human erythropoietin increases hematocrit levels and reduces the need for red blood cell transfusions in chronic kidney disease patients with anemia (1–3). Some patients, however, exhibit persistent or intermittent hyporesponsiveness to erythropoietin therapy and fail to achieve target hemoglobin levels despite receiving large erythropoietin doses (4, 5). Recent studies of hemodialysis patients have found that hyporesponsiveness to erythropoietin therapy is associated with elevated mortality risk (6–9). An improved understanding of factors that influence erythropoietin response could lead to more effective anemia management strategies and improved clinical outcomes.
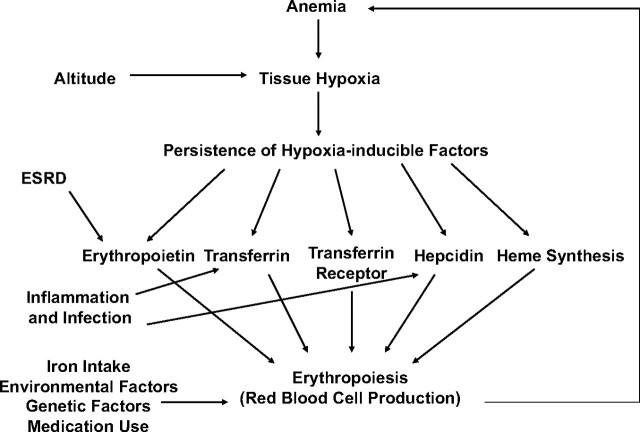
In Figure 1, we propose a simple model of the feedback system that maintains hemoglobin concentrations. System equilibrium is disturbed when the oxygen-carrying capacity of the blood drops enough to activate hypoxia-inducible transcription factors (HIFs), the key regulators of oxygen homeostasis (10–12). In a healthy kidney, activation of HIFs leads to expression of endogenous erythropoietin and subsequent stimulation of erythropoiesis, provided that iron is available (13). HIFs also affect expression of the proteins involved in iron metabolism, such as transferrin, concerned with iron transport, and transferrin receptor, concerned with iron uptake (primarily by developing erythrocytes) (14, 15). HIFs also exert a suppressive effect on hepcidin, a hormone that regulates iron availability and absorption (16, 17).
Figure 1.
Physiologic model of the feedback system that regulates hemoglobin levels. ESRD, end-stage renal disease.
In end-stage renal disease (ESRD), the feedback system depicted in Figure 1 is disrupted because ESRD patients are unable to produce sufficient amounts of erythropoietin to fully correct their anemia. Inflammation also contributes to anemia by down-regulating transferrin and up-regulating hepcidin (16, 18). This process makes iron less accessible to invading microorganisms but limits the iron available for erythropoiesis. Indeed, what has been termed the “anemia of chronic disease” is now thought to be caused primarily by inflammation (19).
Similar to anemia, increased altitude may induce tissue hypoxia because a lowered atmospheric partial pressure of oxygen results in decreased loading of oxygen onto hemoglobin. Therefore, moving to a higher altitude should trigger increased activation of hypoxia-inducible factors in certain cells and thus may influence many HIF-regulated pathways. In healthy people, exposure to altitude leads to increased erythropoiesis and an expansion of red blood cell mass. In ESRD patients, altitude-induced hypoxia has been shown to produce a negligible-to-small increase in endogenous erythropoietin expression by the diseased kidney (20–22) but could have normal effects on the other systems contributing to erythropoiesis, such as those involved with iron metabolism (23).
In a recent study, it was reported that hemodialysis patients living at high altitude use less erythropoietin but achieve higher hematocrit levels than apparently comparable patients living at lower altitudes (24). Given the theoretical effects of hypoxia on iron availability, we hypothesized that the effect of altitude may be particularly strong in ESRD patients, whose response to erythropoietin is limited by the iron sequestration caused by inflammation. To explore this hypothesis, we studied the natural experiment that occurs when dialysis patients move from a low to a higher altitude. Using 13 years of data on all patients in Medicare's ESRD program, we identified a cohort of patients who moved up from a dialysis center located below 2,000 feet (600 m; 1 foot = 0.3 m) to another one located at a higher altitude. We examined how increased altitude affected both achieved hematocrit levels and erythropoietin use. To determine whether the effect of altitude was more pronounced in patients with possible iron sequestration, we compared the effect of altitude change in patients with a lower response to erythropoietin with those with a greater treatment response during the time period before the move.
MATERIALS AND METHODS
Data
We obtained data from the United States Renal Data System (USRDS) and the United States Geological Survey (USGS). The USRDS contains detailed data on all patients in Medicare's ESRD program, including information collected at dialysis initiation (reported on the Medical Evidence Form, CMS-2728), describing demographics, primary cause of ESRD, clinical data (e.g., weight), and certain laboratory measurements (e.g., serum albumin and hematocrit levels). In addition, the USRDS contains all Medicare Part A and B claims that include information on diagnoses and procedures recorded for all hospitalizations and outpatient visits. The USRDS also contains claims for total monthly erythropoietin doses, reported with the final hematocrit level recorded during the month.
From USGS data, we obtained a list of 137,061 US cities along with their state and county Federal Information Processing Standards code and their elevation. We also obtained a list of US zip codes with their primary city and the average elevation of the county, as reported by the USGS. We then matched the zip code data to the USGS city elevation data using city name, county, and state Federal Information Processing Standards code. Doing so enabled us to assign to each zip code the elevation of its primary city. For zip codes that could not be matched to a city in the USGS data, we set the elevation equal to the average elevation in the county. Altitudes were assigned to each dialysis unit based on their zip code as reported in the USRDS facility file.
The investigators obtained Data Use Agreements from the National Institute of Digestive and Diabetes and Kidney Diseases. The Brigham and Women's Hospital Institutional Review Board approved this research.
Patient selection
From the USRDS standard analytic files, we selected all patients who initiated hemodialysis between January 1, 1992, and June 1, 2004. Patients were eligible for the study as long as they remained on hemodialysis. Patients became ineligible if they switched to peritoneal dialysis or received a kidney transplant (because hematocrit levels were no longer consistently available for these patients). Eligibility also ended if patients were lost to follow-up, reached the administrative end of follow-up (December 31, 2004), or died.
Patients were followed up for 12 months after moving to a higher elevation. During each month in which patients were eligible, we used the USGS data to determine the approximate altitude of the dialysis center at which the patient received dialysis. Patients entered the study sample if they received dialysis for 2 consecutive months at a center located below 2,000 feet and then began receiving dialysis at a center located at an elevation 250 feet (75 m) or more higher than the dialysis center at which they were previously treated. Study subjects also had to spend fewer than 5 days in the hospital during the month before the move because erythropoietin dosing information is unavailable for patients while they are in the hospital. From this population, we defined 2 study groups: 1) patients who moved to an altitude of more than 3,000 feet (900 m), and 2) a control group of patients who moved up 250–500 (150 m) feet. The control group was required to move to ensure that the group was not enriched with patients with greater disease severity and who were unable to move.
We converted the total erythropoietin dose administered during each month of follow-up into units per week by multiplying the total monthly dose by 7 and then dividing by the number of outpatient days in the month (total days minus time spent in the hospital during the month). We created 4 strata based on the hematocrit and erythropoietin dosing during the 2-month baseline period before the move. We first stratified the sample on the basis of the lower quintile of hematocrit (<32% and ≥32%). We then created 2 additional strata within each of the hematocrit strata based on the median erythropoietin dose within the strata: 13,700 units/week among patient with a hematocrit of less than 32% and 10,800 units/week among patients with a hematocrit of 32% or greater.
Statistical analysis
To characterize the patient groups, we computed summary statistics for a wide range of variables within strata of hematocrit, erythropoietin dose, and altitude change. For each group of patients, we assessed baseline hematocrit and erythropoietin dosing during the 2-month baseline period. To evaluate the effect of altitude change, we computed the within-patient change in hematocrit and erythropoietin dose for each month during the 12 months of follow-up. Patients were censored at the time of death, transplant receipt, loss to follow-up, or a subsequent move (up or down) of more than 1,000 feet (300 m).
To account for repeated observations within an individual, all statistical analyses evaluating the primary endpoints (change in hematocrit level and change in erythropoietin dose) were based on linear models estimated by using generalized estimating equation methodology. These approaches estimate parameters using an independence-structured working variance-covariance matrix. This approach computes asymptotically correct confidence intervals when the data are dependent (e.g., clustered or repeated-measures data) (25). We used the least-squares-means procedure within generalized estimating equations to estimate population-averaged month-to-month changes in hematocrit levels and erythropoietin doses. These regression models were used to compute population-averaged (least-squares) means and 95% confidence intervals for each statistic. This approach uses the fitted regression model to estimate a mean hematocrit and erythropoietin dose change standardized to the distribution of covariates observed in the overall population.
We conducted several secondary analyses to explore the sensitivity of our results to confounding and informative censoring. To adjust for possible confounding, we included covariates in our regression models of change in both hematocrit and erythropoietin. The covariates included age, sex, race, calendar year, weight, primary recorded cause of ESRD (diabetes, hypertension, glomerulonephritis, other), history of cancer, history of myocardial infarction, erythropoietin use, and hematocrit in the baseline period. To further explore the possibility of confounding, we examined the effect of altitude change within strata of race. To investigate possible issues related to informative censoring, we conducted analyses restricted to patients who moved and remained at the new elevation for at least 6 months. To explore the possibility of a dose-response relation, we conducted an analysis in which the altitude group was redefined to be patients who moved up to an altitude of 1,000–3,000 feet (rather than ≥3000 feet).
All statistical analyses were performed by using SAS version 9.1 software (26).
RESULTS
Between 1992 and 2004, we identified 53,233 instances in which hemodialysis patients who met the study entry requirements moved from a dialysis center located below 2,000 feet to one located at least 250 feet higher. Of these instances, 5,274 involved moves of 3,000 feet or more (the altitude group) and 25,345 involved moves of 250–500 feet (the control group).
Table 1 presents the characteristics of the patients in these 2 groups stratified by hematocrit and average weekly erythropoietin dose before the move. Within each strata, patients in the altitude group were similar to patients in the control group with respect to age, body mass index (at start of dialysis), serum albumin (at start of dialysis), and history of many medical comorbid conditions as reported on the medical evidence form. However, race was strongly imbalanced across groups. Black patients were underrepresented in the altitude group, with 10%–20% being of black race compared with 30%–40% in the control group. Patients in the altitude group were very likely to be censored quickly, mostly because of a move to a lower elevation during follow-up. Of the 5,274 patients in the altitude group, 3,990 were censored 1 month after the move. After 12 months, only 462 patients remained uncensored. Of the 25,345 patients in the control group, only 1,133 were censored at 1 month. By 12 months after the move, 17,743 patients remained uncensored.
Table 1.
Baseline Characteristics of US Subjects in the Sample, by Elevation and Hematocrit Group, 1992–2004
| Characteristic | Low Hematocrit and Low EPO Dose |
Low Hematocrit and High EPO Dose |
High Hematocrit and Low EPO Dose |
High Hematocrit and High EPO Dose |
||||||||||||
| Control Group (n = 2,855; 100%) |
Altitude Group (n = 543; 100%) |
Control Group (n = 2,952; 100%) |
Altitude Group (n = 456; 100%) |
Control Group (n = 9,514; 100%) |
Altitude Group (n = 2,315; 100%) |
Control Group (n = 10,024; 100%) |
Altitude Group (n = 1,960; 100%) |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Male gender | 1,387 | 48.5 | 303 | 55.8 | 1,508 | 51.1 | 244 | 52.3 | 5,147 | 54.1 | 1,311 | 56.6 | 5,114 | 51.0 | 1,085 | 55.4 |
| Age, yearsa | 67.0 | 20 | 67.0 | 20 | 62.1 | 0.2 | 61.7 | 0.5 | 68.0 | 19 | 68 | 17 | 66 | 20 | 67 | 19 |
| Race | ||||||||||||||||
| White | 1,640 | 57.4 | 421 | 77.5 | 1,622 | 55.0 | 328 | 72.0 | 5,934 | 62.4 | 1,821 | 78.7 | 5,713 | 57.0 | 1,514 | 77.0 |
| Black | 1,114 | 49.7 | 61 | 11.2 | 1,239 | 42.0 | 90 | 19.7 | 3,216 | 33.8 | 250 | 10.8 | 3,953 | 39.4 | 511 | 11.9 |
| History of myocardial infarction | 106 | 3.7 | 18 | 3.3 | 158 | 5.3 | 23 | 5.0 | 545 | 5.7 | 133 | 5.7 | 633 | 6.3 | 102 | 5.2 |
| History of cancer | 51 | 1.7 | 10 | 1.8 | 98 | 3.3 | 10 | 2.2 | 351 | 3.7 | 91 | 3.9 | 435 | 4.3 | 80 | 4.1 |
| History of COPD | 66 | 2.3 | 20 | 3.7 | 110 | 3.7 | 17 | 3.7 | 419 | 4.4 | 102 | 4.4 | 484 | 4.8 | 65 | 3.3 |
| History of congestive heart failure | 359 | 12.6 | 58 | 10.7 | 540 | 18.3 | 90 | 19.7 | 1,983 | 20.8 | 438 | 18.9 | 2,387 | 23.8 | 380 | 19.4 |
| History of dysrhythmia | 78 | 2.7 | 12 | 2.2 | 100 | 3.4 | 21 | 4.6 | 397 | 4.2 | 85 | 3.7 | 430 | 4.3 | 84 | 4.3 |
| History of PVD | 136 | 4.7 | 30 | 5.5 | 223 | 7.5 | 30 | 6.6 | 958 | 10.1 | 226 | 9.7 | 1,000 | 10.0 | 213 | 10.9 |
| History of diabetes | 510 | 17.7 | 109 | 20.1 | 800 | 27.1 | 140 | 30.7 | 3,221 | 33.9 | 792 | 34.2 | 3,825 | 38.2 | 730 | 37.2 |
| History of cerebrovascular accident | 107 | 3.6 | 28 | 5.2 | 134 | 4.5 | 19 | 4.2 | 629 | 6.6 | 134 | 5.8 | 656 | 6.5 | 96 | 5.0 |
| History of ischemic heart disease | 249 | 8.7 | 41 | 7.6 | 391 | 13.3 | 53 | 11.6 | 1,733 | 18.2 | 399 | 17.2 | 1,994 | 19.9 | 334 | 17.0 |
| Body mass index, kg/m2a | 25.0 | 7.6 | 23.9 | 6.9 | 26.6 | 9.3 | 26.8 | 9.9 | 25.5 | 7.7 | 25.2 | 7.2 | 26.6 | 8.7 | 26.7 | 8.4 |
| Serum albumin, g/dLa | 3.3 | 0.8 | 3.3 | 0.9 | 3.2 | 0.8 | 3.1 | 0.9 | 3.3 | 0.9 | 3.3 | 0.9 | 3.3 | 0.9 | 3.3 | 0.9 |
Abbreviations: COPD, chronic obstructive pulmonary disease; EPO, erythropoietin; PVD, peripheral vascular disease.
Values are expressed as median (interquartile range).
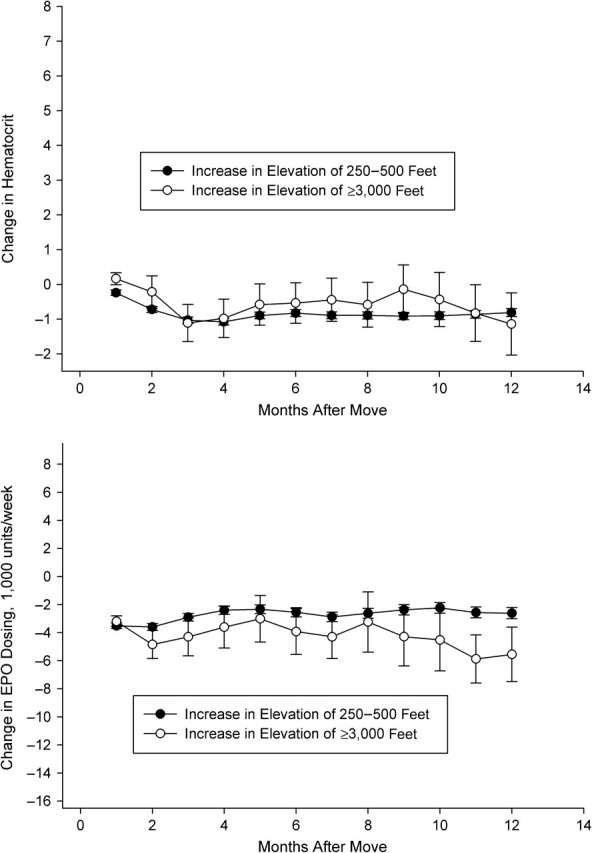
In Figures 2–5, we depict the change in hematocrit (upper panel) and erythropoietin dose (lower panel) for each month during follow-up for the altitude and control groups within each stratum of baseline hematocrit and average weekly erythropoietin use. Across all graphs, we found results consistent with regression to the mean. For example, in patients selected to have low hematocrit, we found increased hematocrit in both groups during follow-up. In patients with hematocrit of less than 32% who received more than the median dose of erythropoietin during the baseline period (patients who exhibited a poor response to erythropoietin during the baseline period), we observed evidence of an altitude effect (Figure 3).
Figure 2.
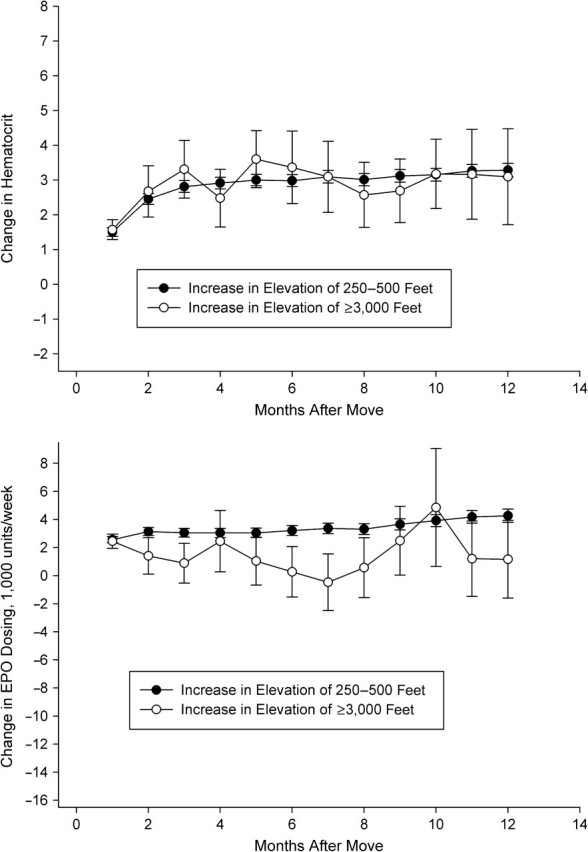
Change in hematocrit (top panel) and erythropoietin (EPO) use (bottom panel), by altitude exposure group, among US patients with hematocrit <32% and average EPO use <13,700 units/week before the altitude change, 1992–2004. One foot = 0.3 m.
Figure 5.
Change in hematocrit (top panel) and erythropoietin (EPO) use (bottom panel), by altitude exposure group, among US patients with hematocrit ≥32% and average EPO use ≥10,800 units/week before the altitude change, 1992–2004. One foot = 0.3 m.
Figure 3.
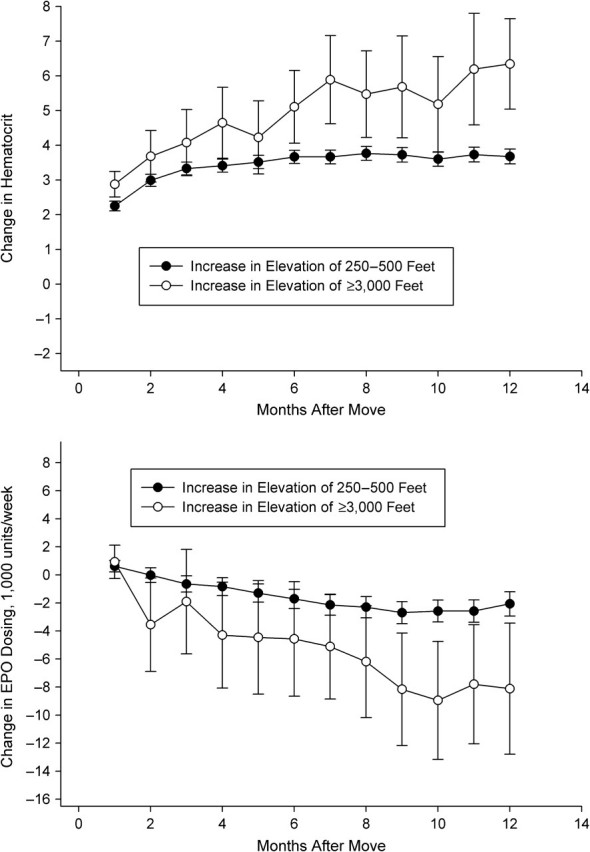
Change in hematocrit (top panel) and erythropoietin (EPO) use (bottom panel), by altitude exposure group, among US patients with hematocrit <32% and average EPO use ≥13,700 units/week before the altitude change, 1992–2004. One foot = 0.3 m.
In these patients, we detected large increases in hematocrit and decreases in erythropoietin dosing in the altitude group relative to the control group. At 6 months, hematocrit had increased in both groups, but more substantially in the altitude group (3.7%, 95% confidence interval: 3.5, 3.9 in the control group vs. 5.1%, 95% confidence interval: 4.1, 6.2 in the altitude group). Erythropoietin dosing decreased in both groups, but by a greater amount in the altitude group (1,700 units/week, 95% confidence interval: 1,000, 2,400 in the control group vs. 4,600 units/week, 95% confidence interval: 500, 8,700 in the altitude group). At 12 months, the differences were more pronounced. Hematocrit had increased similarly in the control group (3.7%, 95% confidence interval: 3.5, 3.9) but by more in the altitude group (6.3%, 95% confidence interval: 5.0, 7.6). Erythropoietin dosing decreased 2,000 units/week (95% confidence interval: 1,200, 2,900) in the control group and 8,100 units/week (95% confidence interval: 3,400, 12,800) in the altitude group. We observed relatively little effect of altitude in the remaining patient subgroups (Figures 2, 4, and 5). There was some evidence that erythropoietin dosing was slightly reduced in patients with low hematocrit and low dose as well as in the patients with high hematocrit and high dose (Figures 2 and 5).
Figure 4.
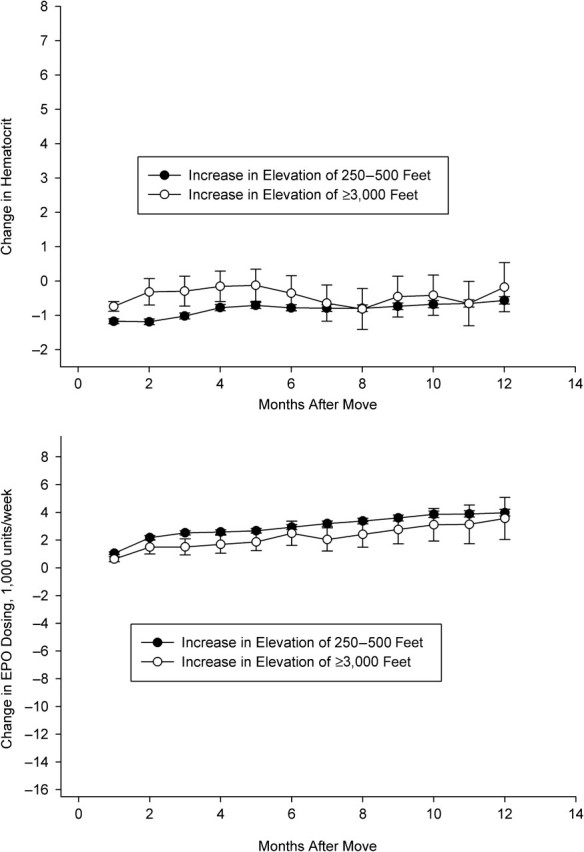
Change in hematocrit (top panel) and erythropoietin (EPO) use (bottom panel), by altitude exposure group, among US patients with hematocrit ≥32% and average EPO use <10,800 units/week before the altitude change, 1992–2004. One foot = 0.3 m.
The sensitivity analysis in which we adjusted for baseline covariates produced results that were quantitatively similar. We also found similar results when the analysis was restricted to white patients. There were insufficient numbers of patients of other race groups to conduct analyses restricted to other races. When we restricted our analysis to patients who were uncensored at 6 months, we observed similar point estimates but larger standard errors for earlier months. Finally, in our analysis comparing patients who moved up 1,000–3,000 feet with patients who moved up only 250–500 feet, we observed a smaller effect of altitude on change in erythropoietin dosing, suggestive of a graded dose-response effect of altitude.
DISCUSSION
We studied the effect of an altitude increase on anemia management and hemoglobin outcomes among maintenance hemodialysis patients. We found that the effect of increased altitude appears to be localized in patients with low hematocrit who received larger erythropoietin doses during the 2-month baseline period. Among these patients, those who moved to a higher altitude and began receiving dialysis at a center located 3,000 feet or more higher than their previous center experienced a greater increase in hematocrit than did similar patients who moved up only 250–500 feet. These same patients also experienced a decrease in erythropoietin dosing relative to the control patients. The changes in hematocrit and erythropoietin dosing were evident after a few months, but they became more pronounced 6–12 months after the altitude change. We found relatively little evidence of an effect of altitude in the remaining patient subgroups, that is, those with higher hematocrit levels or those with low hematocrit levels who were receiving low doses of erythropoietin. These findings are consistent with a previous cross-sectional analysis reporting decreased erythropoietin use but higher achieved hematocrit levels in hemodialysis patients residing at higher altitude (24). The present study builds on the previous research in that it suggests that the effect of altitude is manifest largely in patients who do not respond well to erythropoietin.
Given the theoretical effects of altitude described previously, we speculated that ESRD patients moving to a high altitude would have more iron available for erythropoiesis. Among ESRD patients who exhibit poor response to erythropoietin as a result of sequestration of iron, we hypothesized that an increase in altitude would result in increased release of stored iron and a subsequent improvement in erythropoietin response. In ESRD patients whose response to erythropoietin is not limited by iron availability, we hypothesized that increased altitude would have little effect on erythropoietin responsiveness. These hypotheses are consistent with our observation that an increase in altitude appears to affect primarily patients with low hematocrit levels prior to the move. These are patients who tend to have greater comorbidity burden (27, 28) and higher levels of inflammation (29–31), and who may not respond well to erythropoietin therapy in part because of the sequestration of iron.
We are aware of several limitations of our research. First, we anticipated that the longitudinal study design would result in a “natural experiment.” However, we found that patients who move up 3,000 feet or more are different in various observed ways from those who move up 250–500 feet. Although our results were based on within-person comparisons and were therefore less subject to confounding by static patient characteristics, it is still possible that unmeasured characteristics may confound the analysis. For example, people who move up 3,000 feet or more may be healthier and more likely to experience a large increase in hematocrit. In support of our findings, we note that statistical adjustment for several important observed factors that we would expect to be associated with erythropoietin response did not qualitatively change our results. It is also possible that observed differences between groups is a result of different treatment practices in dialysis centers at higher altitude. However, this would not explain why the differences between groups were observed only in those patients with low hematocrit who were receiving high doses of erythropoietin prior to the move.
Second, it is possible that the effects we observed are not due to altitude-induced hypoxia but are instead a result of environmental factors associated with higher altitude, such as temperature, sunlight intensity, humidity, or concentrations of ambient particulate matter. These possibilities would have to be explored in an analysis that appropriately accounts for such factors. Third, other physiologic changes are known to occur at altitude that could affect our results, including changes in plasma and red cell volume (32, 33). Patients with ESRD have limited ability to regulate plasma volume, but increased red cell volume at altitude could lead to increased hematocrit without any change in erythropoiesis. Nevertheless, if such a phenomenon were occurring, we would expect to find it in all patients, not just those with low hematocrit prior to the move.
Our research was also limited by the absence of iron-related laboratory test results in the database and accurate information about iron dosing. We have speculated that the effect of altitude on anemia and erythropoietin requirements may exist primarily in patients with a functional iron deficiency caused by inflammation. With data that include measures of ferritin and transferrin saturation, this hypothesis could be more directly tested. Without such data, however, we cannot rule out other physiologic mechanisms for the associations we observed.
Given the many potential physiologic effects of altitude in patients with kidney disease (34), there has been an interest in understanding how altitude may affect various clinical outcomes in patients with ESRD (35). The design we propose here could be used along with a conventional cross-sectional analysis to robustly explore the effect of altitude and altitude change on clinical outcomes in hemodialysis patients.
Hypoxia and inflammation are known to be opposing forces affecting erythropoiesis. Inflammation impairs erythropoiesis, whereas hypoxia promotes erythropoiesis. Our results raise the possibility that altitude-induced hypoxia may enhance erythropoietin response in patients who do not respond well to erythropoietin as a result of inflammation. Our findings and related research suggest that erythropoietin hyporesponsiveness could be alleviated through therapeutic interventions that either mimic hypoxia or selectively suppress components of inflammation. Some effects of hypoxia can be induced by pharmacologic stabilization of HIF-1α, the oxygen-sensing subunit of the HIF-1 transcription factor (11). Similarly, a wide variety of compounds are known to exert antiinflammatory effects that may improve erythropoiesis in poorly responsive patients. For example, pharmacologic inhibition of the receptor for interleukin-6, a proinflammatory cytokine that induces hepcidin expression, has been reported to increase hemoglobin levels in patients with rheumatoid arthritis (36). Future research examining the use of erythropoietin in combination with hypoxia-mimicking or inflammation-suppressing therapies may help to identify more effective anemia management strategies for patients with ESRD.
Acknowledgments
Author affiliations: Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (M. Alan Brookhart, Jerry Avorn, Sebastian Schneeweiss, Wolfgang C. Winkelmayer); Department of Epidemiology, UNC Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (M. Alan Brookhart); Department of Biostatistics and Epidemiology, Amgen, Inc., Thousand Oaks, California (Brian D. Bradbury); Department of Epidemiology, School of Public Health, University of California, Los Angeles, Los Angeles, California (Brian D. Bradbury); and Division of Nephrology, Department of Medicine, Stanford University School of Medicine, Palo Alto, California (Wolfgang C. Winkelmayer).
This research was supported by an investigator-initiated research grant from Amgen to the Brigham and Women's Hospital (M. Alan Brookhart, Principal Investigator). Dr. Brookhart's work was also supported by a career development award from the National Institute on Aging (AG027400).
Data reported herein were supplied by the USRDS. Interpretation and reporting of these data are the responsibility of the authors and in no way should be considered official policy or interpretation of the US government.
Dr. Brookhart has received investigator-initiated grant support from Amgen. He has also been a member of advisory boards for Amgen but has not received personal compensation for this activity. Dr. Bradbury is an employee of Amgen. Dr. Winkelmayer has received investigator-initiated grant support from Amgen. He has consulted and sat on advisory boards for Amgen, Fresenius Medical Care (Waltham, Massachusetts), and AMAG Pharmaceuticals (Lexington, Massachusetts).
Glossary
Abbreviations
- ESRD
end-stage renal disease
- HIF
hypoxia-inducible transcription factor
- USGS
United States Geological Survey
- USRDS
United States Renal Data System
References
- 1.Eschbach JW, Egrie JC, Downing MR, et al. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316(2):73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 2.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 3.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 4.Ebben JP, Gilbertson DT, Foley RN, et al. Hemoglobin level variability: associations with comorbidity, intercurrent events, and hospitalizations. Clin J Am Soc Nephrol. 2006;1(6):1205–1210. doi: 10.2215/CJN.01110306. [DOI] [PubMed] [Google Scholar]
- 5.Kausz AT, Solid C, Pereira BJ, et al. Intractable anemia among hemodialysis patients: a sign of suboptimal management or a marker of disease? Am J Kidney Dis. 2005;45(1):136–147. doi: 10.1053/j.ajkd.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 6.Gilbertson DT, Ebben JP, Foley RN, et al. Hemoglobin level variability: associations with mortality. Clin J Am Soc Nephrol. 2008;3(1):133–138. doi: 10.2215/CJN.01610407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilpatrick RD, Critchlow CW, Fishbane S, et al. Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(4):1077–1083. doi: 10.2215/CJN.04601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishani A, Solid CA, Weinhandl ED, et al. Association between number of months below K/DOQI haemoglobin target and risk of hospitalization and death. Nephrol Dial Transplant. 2008;23(5):1682–1689. doi: 10.1093/ndt/gfm845. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury BD, Danese MD, Gleeson M, et al. Effect of Epoetin alfa dose changes on hemoglobin and mortality in hemodialysis patients with hemoglobin levels persistently below 11 g/dL. Clin J Am Soc Nephrol. 2009;4(3):630–637. doi: 10.2215/CJN.03580708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberger C, Rosen S, Shina A, et al. Hypoxia-inducible factors and tubular cell survival in isolated perfused kidneys. Kidney Int. 2006;70(1):60–70. doi: 10.1038/sj.ki.5000395. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell P. HIF-1: an oxygen response system with special relevance to the kidney. J Am Soc Nephrol. 2003;14(11):2712–2722. doi: 10.1097/01.asn.0000092792.97122.e0. [DOI] [PubMed] [Google Scholar]
- 12.Gunaratnam L, Bonventre JV. HIF in kidney disease and development. J Am Soc Nephrol. 2009;20(9):1877–1887. doi: 10.1681/ASN.2008070804. [DOI] [PubMed] [Google Scholar]
- 13.Huang LE, Ho V, Arany Z, et al. Erythropoietin gene regulation depends on heme-dependent oxygen sensing and assembly of interacting transcription factors. Kidney Int. 1997;51(2):548–552. doi: 10.1038/ki.1997.76. [DOI] [PubMed] [Google Scholar]
- 14.Rolfs A, Kvietikova I, Gassmann M, et al. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272(32):20055–20062. doi: 10.1074/jbc.272.32.20055. [DOI] [PubMed] [Google Scholar]
- 15.Lok CN, Ponka P. Identification of a hypoxia response element in the transferrin receptor gene. J Biol Chem. 1999;274(34):24147–24152. doi: 10.1074/jbc.274.34.24147. [DOI] [PubMed] [Google Scholar]
- 16.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz T. Hepcidin and its role in regulating systemic iron metabolism. Hematology/the education program of the American Society of Hematology. Hematology. 2006;29–35:507. doi: 10.1182/asheducation-2006.1.29. [DOI] [PubMed] [Google Scholar]
- 18.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 19.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 20.Blumberg A, Keller H, Marti HR. Effect of altitude on erythropoiesis and oxygen affinity in anaemic patients on maintenance dialysis. Eur J Clin Invest. 1973;3(2):93–97. doi: 10.1111/j.1365-2362.1973.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 21.Bosman DR, Osborne CA, Marsden JT, et al. Erythropoietin response to hypoxia in patients with diabetic autonomic neuropathy and non-diabetic chronic renal failure. Diabet Med. 2002;19(1):65–69. doi: 10.1046/j.1464-5491.2002.00634.x. [DOI] [PubMed] [Google Scholar]
- 22.Quick J, Eichenberger A, Binswanger U. Stimulation of erythropoietin in renal insufficiency by hypobaric hypoxia. Nephrol Dial Transplant. 1992;7(10):1002–1006. [PubMed] [Google Scholar]
- 23.Robach P, Fulla Y, Westerterp KR, et al. Comparative response of EPO and soluble transferrin receptor at high altitude. Med Sci Sports Exerc. 2004;36(9):1493–1498. doi: 10.1249/01.mss.0000139889.56481.e0. discussion 1492. [DOI] [PubMed] [Google Scholar]
- 24.Brookhart MA, Schneeweiss S, Avorn J, et al. The effect of altitude on dosing and response to erythropoietin in ESRD. J Am Soc Nephrol. 2008;19(7):1389–1395. doi: 10.1681/ASN.2007111181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
- 26.SAS Institute . Version 9.1 computer software. Cary, NC: SAS Institute, Inc; 2003. Inc. [Google Scholar]
- 27.Collins AJ, Li S, St Peter W, et al. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39% J Am Soc Nephrol. 2001;12(11):2465–2473. doi: 10.1681/ASN.V12112465. [DOI] [PubMed] [Google Scholar]
- 28.Bradbury BD, Wang O, Critchlow CW, et al. Exploring relative mortality and epoetin alfa dose among hemodialysis patients. Am J Kidney Dis. 2008;51(1):62–70. doi: 10.1053/j.ajkd.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Roberts TL, Obrador GT, St Peter WL, et al. Relationship among catheter insertions, vascular access infections, and anemia management in hemodialysis patients. Kidney Int. 2004;66(6):2429–2436. doi: 10.1111/j.1523-1755.2004.66020.x. [DOI] [PubMed] [Google Scholar]
- 30.Gunnell J, Yeun JY, Depner TA, et al. Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 1999;33(1):63–72. doi: 10.1016/s0272-6386(99)70259-3. [DOI] [PubMed] [Google Scholar]
- 31.Bradbury BD, Critchlow CW, Weir MR, et al. Impact of elevated C-reactive protein levels on erythropoiesis-stimulating agent (ESA) dose and responsiveness in hemodialysis patients. Nephrol Dial Transplant. 2009;24(3):919–925. doi: 10.1093/ndt/gfn543. [DOI] [PubMed] [Google Scholar]
- 32.Weil JV, Jamieson G, Brown DW, et al. The red cell mass–arterial oxygen relationship in normal man. Application to patients with chronic obstructive airway disease. J Clin Invest. 1968;47(7):1627–1639. doi: 10.1172/JCI105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawka MN, Convertino VA, Eichner ER, et al. Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc. 2000;32(2):332–348. doi: 10.1097/00005768-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Luks AM, Johnson RJ, Swenson ER. Chronic kidney disease at high altitude. J Am Soc Nephrol. 2008;19(12):2262–2271. doi: 10.1681/ASN.2007111199. [DOI] [PubMed] [Google Scholar]
- 35.Winkelmayer WC, Liu J, Brookhart MA. Altitude and all-cause mortality in incident dialysis patients. JAMA. 2009;301(5):508–512. doi: 10.1001/jama.2009.84. [DOI] [PubMed] [Google Scholar]
- 36.Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67(11):1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]