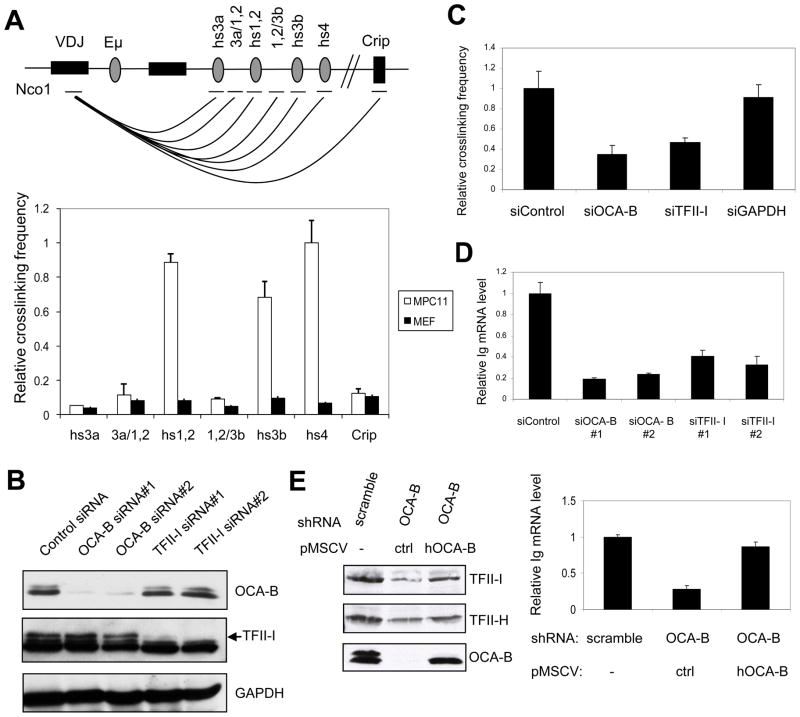
Figure 4.
OCA-B and TFII-I are required for promoter-enhancer interaction and Igh gene transcription. (A) Schematic diagram of the murine Igh locus in MPC11 cells (top) and relative interaction frequencies between Igh promoter and 3′enhancer elements as measured by 3C analysis (bottom). A very low interaction frequency was detected between VH and the Crip gene 30kb downstream of the 3′enhancers. (B) siRNA-mediated knockdown of OCA-B or TFII-I reduces the endogenous Igh transcription level in MPC11 cells. MPC11 cells were treated either with OCA-B or TFII-I siRNAs or a non-specific (control) siRNA and whole cell extracts were analyzed by immunoblot. (C) Effects of OCA-B and TFII-I knockdowns on endogenous Igh promoter-HS4 enhancer interactions in MPC11 cells. Interactions were measured by 3C analysis as described in Methods. (D) Effects of OCA-B and TFII-I knockdowns on endogenous Igh transcription in MPC11 cells. Steady state Igh mRNA levels were analyzed by real-time RT-PCR and normalized to 18S rRNA levels. (E) Rescue of the effects of OCA-B knockdown by overexpression of human OCA-B. MPC11 cells were treated either with control or OCA-B shRNAs and, in the latter case, infected with control or human OCA-B–expressing retroviruses. Whole cell extracts were analyzed by immunoblot (left panel) and Igh mRNA levels were analyzed by qRT-PCR and normalized to 18S rRNA levels (right panel). Data are presented as the mean ± SD (A,C,D,E).