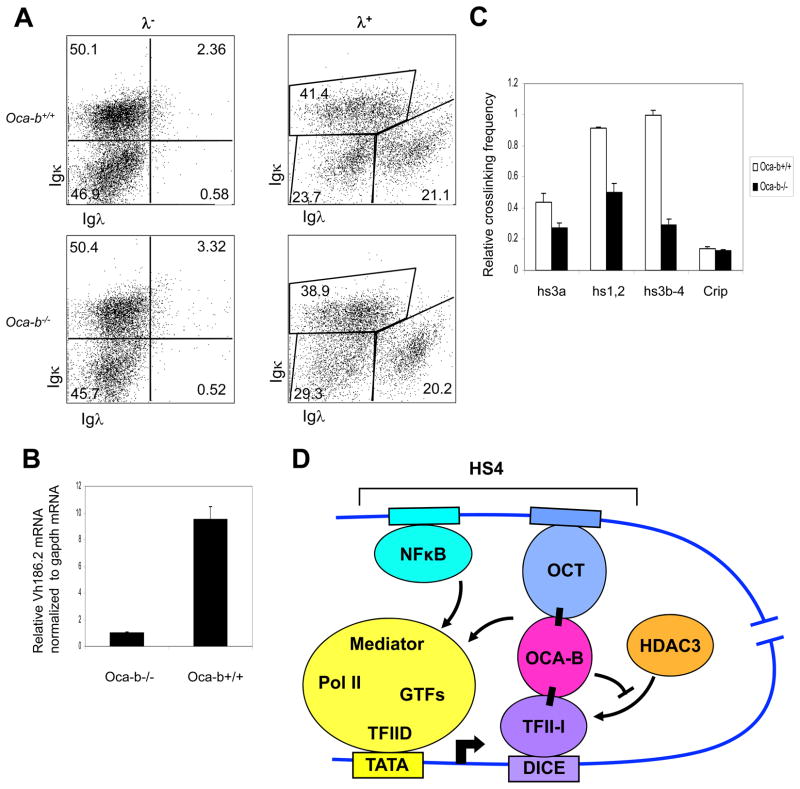
Figure 7.
OCA-B requirement for endogenous Igh expression in primary B cells. (A) Cellular populations from NP-immunized Oca-b+/+ and Oca-b−/− splenocytes were analyzed by FACS using antibodies against the indicated cell surface markers. (B) VH186.2 mRNA levels in Oca-b+/+ and Oca-b−/− splenocytes were analyzed by quantitative RT-PCR after normalization to Gapdh mRNA. Results are presented as the mean ± SD. (C) Effects of OCA-B on Igh promoter-enhancer interactions in wild type and OCA-B null primary cells were measured by 3C as described in Methods. After HindIII digestion, all Igh genes containing JH2 or JH3 segments after VDJ recombination were analyzed. (D) A proposed model for OCA-B and TFII-I interaction that facilitates Igh promoter and HS4 enhancer communication. First, binding of OCA-B to promoter-bound TFII-I relieves HDAC3 binding and the associated repression. Second, an interaction between enhancer-bound OCA-B and TFII-I facilitates juxtaposition, by looping, between Igh promoter and enhancer elements. Proper enhancer function is achieved by facilitated factor recruitment to the promoter and/or by a direct transfer mechanism.