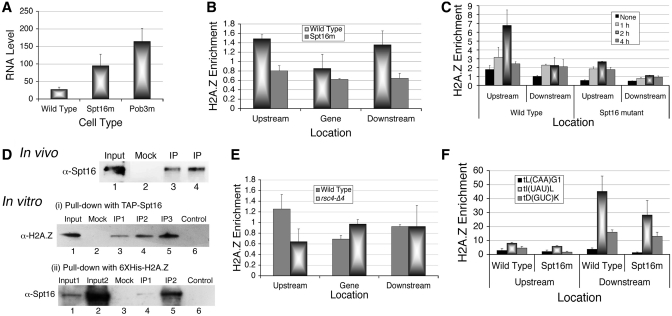
Figure 5.
FACT is a H2A.Z chaperone. (A) In vivo analysis of SUP4 expression in mutants of Spt16 and Pob3. Total RNA was isolated and intron-specific primer extension products of SUP4 were visualized by phosphorImaging of the gel as described under methods. RNA levels were normalized against U4 and average from three independent experiments with scatter are shown. (B) Relative enrichment of HA-tagged H2A.Z in the absence of functional Spt16 in active condition. Comparative levels of H2A.Z are low in both the nucleosomes in Spt16 mutant cell. (C) HA-H2A.Z levels around the SUP4 gene in wild type cells change with time in parrallel to transcriptional repression under nutrient starvation. (D) Co-immunoprecipitation of H2A.Z and Spt16 in vivo and in vitro. Mock was as described under Supplementary Data. Input lanes are loaded with one-third (in vivo) or one-fourth (in vitro) of the sample as compared to IP (immunoprecipitate) lanes. Spt16 was at increasing amounts in IP1, 2 and 3. Upper panel: In vivo, H2A.Z-HA is immunoprecipitated with anti-HA antibody and IP is probed with anti-Spt16 antibody. Lanes 3 and 4 are the duplicates of the immunoprecipitation (IP). Lower panel: (i) and (ii) show in vitro pull-downs, using purified Spt16/Pob3 or 6XHis-tagged H2A.Z; probed with anti-H2A.Z or anti-Spt16 antibodies, respectively. Control shows the immobilized bait protein alone on beads while mock lanes show incubation of the prey with bare beads. (E) Relative enrichment of HA-tagged H2A.Z in rsc4-Δ4 mutant in active condition. (F) Relative enrichment of H2A.Z-HA on tDNA loci present on three different chromosomes.