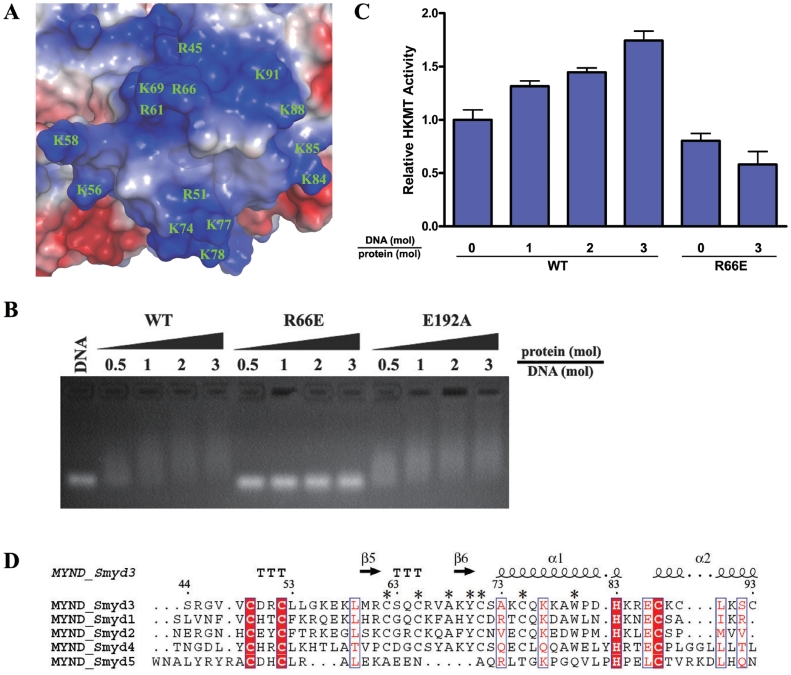
Figure 5.
Modulation of the HKMT activity of Smyd3 via DNA binding of the MYND Domain. (A) Electrostatic potential surface of the MYND domain with the negative charge in red and the positive charge in blue. All the positively charged residues are labeled. (B) Analysis of the interaction between the wild-type and mutant Smyd3 proteins and the potential target DNA with gel shift assays. The wild-type (WT) Smyd3 and the R66E and E192A mutants were loaded to 1 µg DNA with different molar ratios as indicated. DNA binding of the wild-type and mutant Smyd3 were analyzed with agarose gel electrophoresis. (C) HKMT assays of the wild-type and R66E mutant Smyd3 in the presence and absence of the potential target DNA. The HKMT activity of the wild-type but not the mutant Smyd3 is stimulated when the potential target DNA is supplemented in the HKMT reaction system. (D) Sequence alignment of the MYND domain among all human Smyd proteins. The sequence number and secondary structure elements of Smyd3 are marked. The invariant residues across these proteins are denoted with filled red boxes, and the highly conserved ones in open boxes. The residues conserved in Smyd1-4 but not Smyd5 are denoted with asterisks.