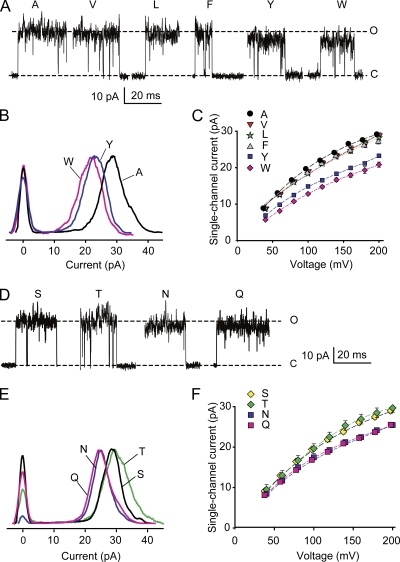
Figure 3.
Increasing side-chain volume at the entrance to the inner cavity reduces iout for both hydrophobic and uncharged hydrophilic amino acids. (A) Representative single-channel currents at +200 mV for substitution of the indicated hydrophobic amino acids at positions 321/324. Dashed lines show the mean open and closed current levels of E321A/E324A channels. Larger side-chain amino acids at positions 321/324 decrease iout. (B) Representative all-points histograms for currents like those in A for the indicated amino acid substitutions. The values on the ordinate are proportional to the frequency of observations of the current levels indicated on the abscissa. The distributions centered on 0 pA indicate the current fluctuation around the closed level, and the distributions to the right indicate the current fluctuations around the open levels. Histograms are scaled so that the peak frequencies of the open current levels are the same. The larger amino acid side chains of tyrosine and tryptophan shift the entire distributions to lower current levels compared with alanine. The excess open-channel noise compared with wt channels (Fig. 2 A) suggests that the substitutions destabilize the open state. (C) Plots of iout versus voltage indicate that the reduction of iout for the larger side chains occurs over the range of examined voltages. Substituting valine, leucine, or phenylalanine for alanine had little effect on iout, whereas substituting tyrosine or tryptophan with their larger side chains reduced iout. (D–F) Same as A–C, except for substitutions of hydrophilic uncharged amino acids. Dashed lines in D are for serine substitution. Asparagine and glutamine with their larger side chains decrease single-channel current compared with serine and threonine with their smaller side chains. For i-V plots in this and Fig. 4, some symbols have been shifted laterally 2.5 mV to avoid overlap. 150 mM K+i.